Assessment of the role of FGF15 in mediating the metabolic outcomes of murine Vertical Sleeve Gastrectomy (VSG)
- PMID: 32967428
- PMCID: PMC7792670
- DOI: 10.1152/ajpgi.00175.2020
Assessment of the role of FGF15 in mediating the metabolic outcomes of murine Vertical Sleeve Gastrectomy (VSG)
Abstract
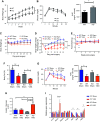
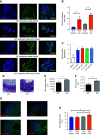
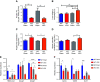
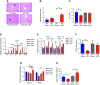
Vertical sleeve gastrectomy (VSG) is the best current therapy for remission of obesity and its co-morbidities. It is understood to alter the enterohepatic circulation of bile acids in vivo. Fibroblast growth factor 19 (FGF19) in human and its murine orthologue Fgf15 plays a pivotal role in this bile acid driven enterohepatic signaling. The present study evaluated the metabolic outcomes of VSG in Fgf15 deficient mice. 6-8 weeks old male wildtype mice (WT) and Fgf15 deficient mice (KO) were fed a high fat diet (HFD) for 8 weeks. At 8th week of diet, both WT and KO mice were randomly distributed to VSG or sham surgery. Post-surgery, mice were observed for 8 weeks while fed a HFD and then euthanized to collect tissues for experimental analysis. Fgf15 deficient (KO) mice lost weight post VSG, but glucose tolerance in KO mice did not improve post VSG compared to WT mice. Enteroids derived from WT and KO mice proliferated with bile acid exposure in vitro. Post VSG both WT and KO mice had similarly altered bile acid enterohepatic flux, however Fgf15 deficient mice post VSG had increased hepatic accumulation of free and esterified cholesterol leading to lipotoxicity related ER stress, inflammasome activation, and increased Fgf21 expression. Intact Fgf15 mediated enterohepatic bile acid signaling, but not changes in bile acid flux, appear to be important for the metabolic improvements post-murine bariatric surgery. These novel data introduce a potential point of distinction between bile acids acting as ligands compared to their canonical downstream signaling pathways.
Keywords: Bile acid; bariatric surgery; lipidomics; liver physiology; obesity.
Conflict of interest statement
R.J.S. has received research support from Novo Nordisk, Zafgen, MedImmune, and Sanofi and has served as a paid consultant to Ethicon Endo-Surgery/Johnson & Johnson, Orexigen, Novo Nordisk, Daiichi Sankyo, Janssen/Johnson & Johnson, Novartis, Paul Hastings Law Firm, Scohia, and Kallyope Inc. D.A.S. receives research support from Novo Nordisk, Ethicon Endo-Surgery, and Zafgen. R.K. receives research support from Johnson & Johnson. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures





Similar articles
-
Intestinal-derived FGF15 protects against deleterious effects of vertical sleeve gastrectomy in mice.Nat Commun. 2021 Aug 6;12(1):4768. doi: 10.1038/s41467-021-24914-y. Nat Commun. 2021. PMID: 34362888 Free PMC article.
-
Vertical sleeve gastrectomy improves glucose-insulin homeostasis by enhancing β-cell function and survival via FGF15/19.Am J Physiol Endocrinol Metab. 2024 Feb 1;326(2):E134-E147. doi: 10.1152/ajpendo.00218.2023. Epub 2023 Dec 20. Am J Physiol Endocrinol Metab. 2024. PMID: 38117265
-
Lipocalin-type prostaglandin D2 synthase (L-PGDS) modulates beneficial metabolic effects of vertical sleeve gastrectomy.Surg Obes Relat Dis. 2016 Sep-Oct;12(8):1523-1531. doi: 10.1016/j.soard.2016.04.007. Epub 2016 Apr 7. Surg Obes Relat Dis. 2016. PMID: 27425837
-
Targeting FXR and FGF19 to Treat Metabolic Diseases-Lessons Learned From Bariatric Surgery.Diabetes. 2018 Sep;67(9):1720-1728. doi: 10.2337/dbi17-0007. Diabetes. 2018. PMID: 30135133 Free PMC article. Review.
-
Bile acids and bariatric surgery.Mol Aspects Med. 2017 Aug;56:75-89. doi: 10.1016/j.mam.2017.04.001. Epub 2017 Apr 17. Mol Aspects Med. 2017. PMID: 28390813 Free PMC article. Review.
Cited by
-
Bile Acid Signaling in Metabolic and Inflammatory Diseases and Drug Development.Pharmacol Rev. 2024 Oct 16;76(6):1221-1253. doi: 10.1124/pharmrev.124.000978. Pharmacol Rev. 2024. PMID: 38977324 Review.
-
Increase in bile acids after sleeve gastrectomy improves metabolism by activating GPBAR1 to increase cAMP in mice with nonalcoholic fatty liver disease.Immun Inflamm Dis. 2024 Jul;12(7):e1149. doi: 10.1002/iid3.1149. Immun Inflamm Dis. 2024. PMID: 39031498 Free PMC article.
-
Gut Factors Mediating the Physiological Impact of Bariatric Surgery.Curr Diab Rep. 2022 Aug;22(8):371-383. doi: 10.1007/s11892-022-01478-9. Epub 2022 Jun 20. Curr Diab Rep. 2022. PMID: 35723770 Free PMC article. Review.
-
Dynamics of the human bile acid metabolome during weight loss.Sci Rep. 2024 Oct 28;14(1):25743. doi: 10.1038/s41598-024-75831-1. Sci Rep. 2024. PMID: 39468179 Free PMC article.
-
The role of the gut-brain axis in bariatric surgery.Curr Opin Neurobiol. 2025 Jun;92:103041. doi: 10.1016/j.conb.2025.103041. Epub 2025 May 10. Curr Opin Neurobiol. 2025. PMID: 40349608 Review.
References
-
- Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, Caron S, Torpier G, Fruchart JC, Gonzalez FJ, Kuipers F, Staels B. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem 281: 11039–11049, 2006. doi:10.1074/jbc.M510258200. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

