Avoiding misdiagnosis: expert consensus recommendations for the suspicion and diagnosis of transthyretin amyloidosis for the general practitioner
- PMID: 32967612
- PMCID: PMC7513485
- DOI: 10.1186/s12875-020-01252-4
Avoiding misdiagnosis: expert consensus recommendations for the suspicion and diagnosis of transthyretin amyloidosis for the general practitioner
Abstract
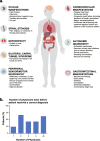
Background: Transthyretin amyloidosis (also known as ATTR amyloidosis) is a systemic, life-threatening disease characterized by transthyretin (TTR) fibril deposition in organs and tissue. A definitive diagnosis of ATTR amyloidosis is often a challenge, in large part because of its heterogeneous presentation. Although ATTR amyloidosis was previously considered untreatable, disease-modifying therapies for the treatment of this disease have recently become available. This article aims to raise awareness of the initial symptoms of ATTR amyloidosis among general practitioners to facilitate identification of a patient with suspicious signs and symptoms.
Methods: These consensus recommendations for the suspicion and diagnosis of ATTR amyloidosis were developed through a series of development and review cycles by an international working group comprising key amyloidosis specialists. This working group met to discuss the barriers to early and accurate diagnosis of ATTR amyloidosis and develop a consensus recommendation through a thorough search of the literature performed using PubMed Central.
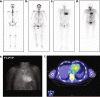
Results: The cardiac and peripheral nervous systems are most frequently involved in ATTR amyloidosis; however, many patients often also experience gastrointestinal and other systemic manifestations. Given the multisystemic nature of symptoms, ATTR amyloidosis is often misdiagnosed as a more common disorder, leading to significant delays in the initiation of treatment. Although histologic evaluation has been the gold standard to confirm ATTR amyloidosis, a range of tools are available that can facilitate early and accurate diagnosis. Of importance, genetic testing should be considered early in the evaluation of a patient with unexplained peripheral neuropathy.
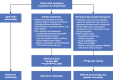
Conclusions: A diagnostic algorithm based on initial red flag symptoms and manifestations of cardiac or neurologic involvement will facilitate identification by the general practitioner of a patient with clinically suspicious symptoms, enabling subsequent referral of the patient to a multidisciplinary specialized medical center.
Keywords: ATTR amyloidosis; ATTRv; Cardiomyopathy; Diagnosis; Polyneuropathy; Transthyretin amyloidosis; hATTR.
Conflict of interest statement
MG reports personal fees from Ionis/Akcea, Alnylam, Prothena, Celgene, Janssen, Spectrum, Annexon, Appellis, Amgen, Medscape, Physicians Education Resource, AbbVie (Data Safety Monitoring board), Research to Practice; speaker fees from Teva, Johnson and Johnson, Medscape, DAVA Oncology; advisory boards for Pharmacyclics and Proclara; royalties from Springer Publishing; and grant funding from Amyloidosis Foundation, International Waldenstrom Foundation, Spectrum, and the National Cancer Institute (SPORE MM SPORE 5P50 CA186781–04).
DA reports grants from Alnylam and Pfizer and personal fees from Prothena, GlaxoSmithKline, Alnylam, and Pfizer.
YA, JMB, and PNH have nothing to disclose.
SB reports personal fees from Pfizer.
TC was paid per protocol for clinical trials from FoldRx, Pfizer, Ionis, and Alnylam and received grants from FoldRx and Pfizer; received support from Pfizer, Ionis, Biogen, and Alnylam to attend scientific meetings; and has presented on behalf of Pfizer, Alnylam, GlaxoSmithKline, Prothena, and Ionis/Akcea and received honoraria.
RLC reports consulting fees from Prothena, Janssen, Amgen, Takeda, Sanofi-Aventis, Unum, Caelum and reports grant/research support from Prothena, Janssen, Takeda, and Karyopharm.
TD reports grants from Pfizer and Alnylam Pharmaceuticals and reports personal fees from Ionis Pharmaceuticals, Prothena Group, and GlaxoSmithKline.
SD reports personal fees from Pfizer, GE Healthcare, and Advanced Accelerator Applications.
BMD reports personal fees from Pfizer and Alnylam Pharmaceuticals.
MF reports a grant from GlaxoSmithKline and reports personal fees from British Heart Foundation, Pfizer, Alnylam Pharmaceuticals, and Prothena.
JDG participated in advisory boards for Alnylam Pharmaceuticals and GlaxoSmithKline.
MG reports grants from Eidos Therapeutics, Pfizer, Alnylam Pharmaceuticals, and Prothena.
IL has received honoraria from Akcea Therapeutics.
AVK reports personal fees from Akcea Therapeutics, Eidos Therapeutics, Pfizer, Alnylam Pharmaceuticals.
FLR reports grants from Pfizer and personal fees from Pfizer and Alnylam Pharmaceuticals.
OBS received honoraria and travel and consultancy fees from Ionis/Akcea, Alnylam, Prothena, and Intellia.
MSM reports grants from Pfizer and Alnylam Pharmaceuticals and reports personal fees from Akcea Therapeutics, Ionis Pharmaceuticals, Prothena Corp, Pfizer, Alnylam Pharmaceuticals, Eidos Therapeutics, and GlaxoSmithKline.
JNN reports grants from Pfizer, Eidos Therapeutics, and Akcea Therapeutics and personal fees from Pfizer, Akcea Therapeutics, Alnylam Pharmaceuticals, and Ionis Pharmaceuticals.
CCQ reports personal fees from Alnylam Pharmaceuticals.
CR reports grants from Pfizer and personal fees from Pfizer and Alnylam Pharmaceuticals.
RW reports grants from Pfizer and Eidos Therapeutics and reports personal fees from Alnylam Pharmaceuticals, Pfizer, and Eidos Therapeutics.
GM has received honoraria from Janssen and Prothena; travel support from Prothena and Celgene; and consulting fees from Millennium, Pfizer, Janssen, Prothena, and Ionis.
Figures
References
-
- Coelho T, Ericzon BG, Falk R, Grogen D. A Physician's guide to Transthyretin amyloidosis. 2008.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

