Ketamine normalizes high-gamma power in the anterior cingulate cortex in a rat chronic pain model
- PMID: 32967695
- PMCID: PMC7513294
- DOI: 10.1186/s13041-020-00670-w
Ketamine normalizes high-gamma power in the anterior cingulate cortex in a rat chronic pain model
Abstract
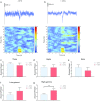
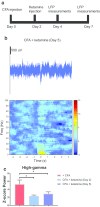
Chronic pain alters cortical and subcortical plasticity, causing enhanced sensory and affective responses to peripheral nociceptive inputs. Previous studies have shown that ketamine had the potential to inhibit abnormally amplified affective responses of single neurons by suppressing hyperactivity in the anterior cingulate cortex (ACC). However, the mechanism of this enduring effect has yet to be understood at the network level. In this study, we recorded local field potentials from the ACC of freely moving rats. Animals were injected with complete Freund's adjuvant (CFA) to induce persistent inflammatory pain. Mechanical stimulations were administered to the hind paw before and after CFA administration. We found a significant increase in the high-gamma band (60-100 Hz) power in response to evoked pain after CFA treatment. Ketamine, however, reduced the high-gamma band power in response to evoked pain in CFA-treated rats. In addition, ketamine had a sustained effect on the high-gamma band power lasting up to five days after a single dose administration. These results demonstrate that ketamine has the potential to alter maladaptive neural responses in the ACC induced by chronic pain.
Keywords: Anterior cingulate cortex; Chronic pain; Gamma band power; Ketamine; Local field potential.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

