Diverse Energy-Conserving Pathways in Clostridium difficile: Growth in the Absence of Amino Acid Stickland Acceptors and the Role of the Wood-Ljungdahl Pathway
- PMID: 32967909
- PMCID: PMC7515248
- DOI: 10.1128/JB.00233-20
Diverse Energy-Conserving Pathways in Clostridium difficile: Growth in the Absence of Amino Acid Stickland Acceptors and the Role of the Wood-Ljungdahl Pathway
Abstract
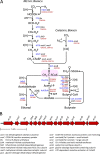
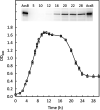
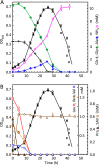
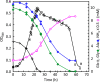
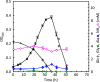
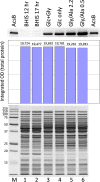
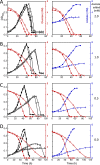
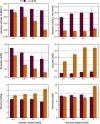
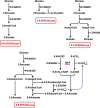
Clostridium difficile is the leading cause of hospital-acquired antibiotic-associated diarrhea and is the only widespread human pathogen that contains a complete set of genes encoding the Wood-Ljungdahl pathway (WLP). In acetogenic bacteria, synthesis of acetate from 2 CO2 molecules by the WLP functions as a terminal electron accepting pathway; however, C. difficile contains various other reductive pathways, including a heavy reliance on Stickland reactions, which questions the role of the WLP in this bacterium. In rich medium containing high levels of electron acceptor substrates, only trace levels of key WLP enzymes were found; therefore, conditions were developed to adapt C. difficile to grow in the absence of amino acid Stickland acceptors. Growth conditions were identified that produce the highest levels of WLP activity, determined by Western blot analyses of the central component acetyl coenzyme A synthase (AcsB) and assays of other WLP enzymes. Fermentation substrate and product analyses, enzyme assays of cell extracts, and characterization of a ΔacsB mutant demonstrated that the WLP functions to dispose of metabolically generated reducing equivalents. While WLP activity in C. difficile does not reach the levels seen in classical acetogens, coupling of the WLP to butyrate formation provides a highly efficient system for regeneration of NAD+ "acetobutyrogenesis," requiring only low flux through the pathways to support efficient ATP production from glucose oxidation. Additional insights redefine the amino acid requirements in C. difficile, explore the relationship of the WLP to toxin production, and provide a rationale for colocalization of genes involved in glycine synthesis and cleavage within the WLP operon.IMPORTANCEClostridium difficile is an anaerobic, multidrug-resistant, toxin-producing pathogen with major health impacts worldwide. It is the only widespread pathogen harboring a complete set of Wood-Ljungdahl pathway (WLP) genes; however, the role of the WLP in C. difficile is poorly understood. In other anaerobic bacteria and archaea, the WLP can operate in one direction to convert CO2 to acetic acid for biosynthesis or in either direction for energy conservation. Here, conditions are defined in which WLP levels in C. difficile increase markedly, functioning to support metabolism of carbohydrates. Amino acid nutritional requirements were better defined, with new insight into how the WLP and butyrate pathways act in concert, contributing significantly to energy metabolism by a mechanism that may have broad physiological significance within the group of nonclassical acetogens.
Keywords: CO dehydrogenase; CODH; Clostridium difficile; Stickland reaction; Wood-Ljungdahl pathway; acetate; acetyl-CoA synthase; acsB; butyrate; carbon monoxide dehydrogenase.
Figures


Similar articles
-
Heterologous Expression of the Clostridium carboxidivorans CO Dehydrogenase Alone or Together with the Acetyl Coenzyme A Synthase Enables both Reduction of CO2 and Oxidation of CO by Clostridium acetobutylicum.Appl Environ Microbiol. 2017 Aug 1;83(16):e00829-17. doi: 10.1128/AEM.00829-17. Print 2017 Aug 15. Appl Environ Microbiol. 2017. PMID: 28625981 Free PMC article.
-
Functional Expression of the Clostridium ljungdahlii Acetyl-Coenzyme A Synthase in Clostridium acetobutylicum as Demonstrated by a Novel In Vivo CO Exchange Activity En Route to Heterologous Installation of a Functional Wood-Ljungdahl Pathway.Appl Environ Microbiol. 2018 Mar 19;84(7):e02307-17. doi: 10.1128/AEM.02307-17. Print 2018 Apr 1. Appl Environ Microbiol. 2018. PMID: 29374033 Free PMC article.
-
Insights into CO2 Fixation Pathway of Clostridium autoethanogenum by Targeted Mutagenesis.mBio. 2016 May 24;7(3):e00427-16. doi: 10.1128/mBio.00427-16. mBio. 2016. PMID: 27222467 Free PMC article.
-
Redox, haem and CO in enzymatic catalysis and regulation.Biochem Soc Trans. 2012 Jun 1;40(3):501-7. doi: 10.1042/BST20120083. Biochem Soc Trans. 2012. PMID: 22616859 Free PMC article. Review.
-
Metabolism the Difficile Way: The Key to the Success of the Pathogen Clostridioides difficile.Front Microbiol. 2019 Feb 15;10:219. doi: 10.3389/fmicb.2019.00219. eCollection 2019. Front Microbiol. 2019. PMID: 30828322 Free PMC article. Review.
Cited by
-
Physiological role and complex regulation of O2-reducing enzymes in the obligate anaerobe Clostridioides difficile.mBio. 2024 Oct 16;15(10):e0159124. doi: 10.1128/mbio.01591-24. Epub 2024 Aug 27. mBio. 2024. PMID: 39189748 Free PMC article.
-
Genome-based metabolic and phylogenomic analysis of three Terrisporobacter species.PLoS One. 2023 Oct 10;18(10):e0290128. doi: 10.1371/journal.pone.0290128. eCollection 2023. PLoS One. 2023. PMID: 37816002 Free PMC article.
-
Isolation and characterization of novel acetogenic strains of the genera Terrisporobacter and Acetoanaerobium.Front Microbiol. 2024 Jul 3;15:1426882. doi: 10.3389/fmicb.2024.1426882. eCollection 2024. Front Microbiol. 2024. PMID: 39021630 Free PMC article.
-
Reconsidering the in vivo functions of Clostridial Stickland amino acid fermentations.Anaerobe. 2022 Aug;76:102600. doi: 10.1016/j.anaerobe.2022.102600. Epub 2022 Jun 13. Anaerobe. 2022. PMID: 35709938 Free PMC article. Review.
-
What's a Biofilm?-How the Choice of the Biofilm Model Impacts the Protein Inventory of Clostridioides difficile.Front Microbiol. 2021 Jun 10;12:682111. doi: 10.3389/fmicb.2021.682111. eCollection 2021. Front Microbiol. 2021. PMID: 34177868 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

