Macrophage secretion of miR-106b-5p causes renin-dependent hypertension
- PMID: 32968066
- PMCID: PMC7511948
- DOI: 10.1038/s41467-020-18538-x
Macrophage secretion of miR-106b-5p causes renin-dependent hypertension
Abstract
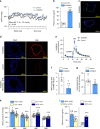
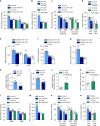
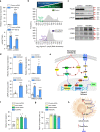
Myeloid cells are known mediators of hypertension, but their role in initiating renin-induced hypertension has not been studied. Vitamin D deficiency causes pro-inflammatory macrophage infiltration in metabolic tissues and is linked to renin-mediated hypertension. We tested the hypothesis that impaired vitamin D signaling in macrophages causes hypertension using conditional knockout of the myeloid vitamin D receptor in mice (KODMAC). These mice develop renin-dependent hypertension due to macrophage infiltration of the vasculature and direct activation of renal juxtaglomerular (JG) cell renin production. Induction of endoplasmic reticulum stress in knockout macrophages increases miR-106b-5p secretion, which stimulates JG cell renin production via repression of transcription factors E2f1 and Pde3b. Moreover, in wild-type recipient mice of KODMAC/miR106b-/- bone marrow, knockout of miR-106b-5p prevents the hypertension and JG cell renin production induced by KODMAC macrophages, suggesting myeloid-specific, miR-106b-5p-dependent effects. These findings confirm macrophage miR-106b-5p secretion from impaired vitamin D receptor signaling causes inflammation-induced hypertension.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- De Ciuceis C, et al. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler. Thromb. Vasc. Biol. 2005;25:2106–2113. doi: 10.1161/01.ATV.0000181743.28028.57. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

