Injectable pre-cultured tissue modules catalyze the formation of extensive functional microvasculature in vivo
- PMID: 32968145
- PMCID: PMC7511337
- DOI: 10.1038/s41598-020-72576-5
Injectable pre-cultured tissue modules catalyze the formation of extensive functional microvasculature in vivo
Abstract
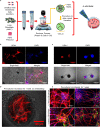
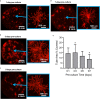
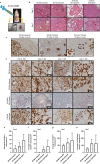
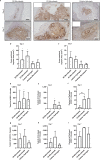
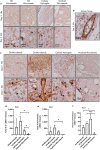
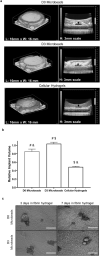
Revascularization of ischemic tissues is a major barrier to restoring tissue function in many pathologies. Delivery of pro-angiogenic factors has shown some benefit, but it is difficult to recapitulate the complex set of factors required to form stable vasculature. Cell-based therapies and pre-vascularized tissues have shown promise, but the former require time for vascular assembly in situ while the latter require invasive surgery to implant vascularized scaffolds. Here, we developed cell-laden fibrin microbeads that can be pre-cultured to form primitive vascular networks within the modular structures. These microbeads can be delivered in a minimally invasive manner and form functional microvasculature in vivo. Microbeads containing endothelial cells and stromal fibroblasts were pre-cultured for 3 days in vitro and then injected within a fibrin matrix into subcutaneous pockets on the dorsal flanks of SCID mice. Vessels deployed from these pre-cultured microbeads formed functional connections to host vasculature within 3 days and exhibited extensive, mature vessel coverage after 7 days in vivo. Cellular microbeads showed vascularization potential comparable to bulk cellular hydrogels in this pilot study. Furthermore, our findings highlight some potentially advantageous characteristics of pre-cultured microbeads, such as volume preservation and vascular network distribution, which may be beneficial for treating ischemic diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Pre-cultured, cell-encapsulating fibrin microbeads for the vascularization of ischemic tissues.J Biomed Mater Res A. 2024 Apr;112(4):549-561. doi: 10.1002/jbm.a.37580. Epub 2023 Jun 16. J Biomed Mater Res A. 2024. PMID: 37326361 Free PMC article.
-
Endothelial sprouting and network formation in collagen- and fibrin-based modular microbeads.Acta Biomater. 2016 Jan;29:33-41. doi: 10.1016/j.actbio.2015.10.022. Epub 2015 Oct 23. Acta Biomater. 2016. PMID: 26481042 Free PMC article.
-
Distributed vasculogenesis from modular agarose-hydroxyapatite-fibrinogen microbeads.Acta Biomater. 2017 Jun;55:144-152. doi: 10.1016/j.actbio.2017.03.050. Epub 2017 Mar 29. Acta Biomater. 2017. PMID: 28365482 Free PMC article.
-
Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering.Acta Biomater. 2019 Sep 1;95:348-356. doi: 10.1016/j.actbio.2019.02.046. Epub 2019 Mar 1. Acta Biomater. 2019. PMID: 30831326
-
Isolation of ready-made rat microvessels and its applications in effective in vivo vascularization and in angiogenic studies in vitro.Nat Protoc. 2022 Dec;17(12):2721-2738. doi: 10.1038/s41596-022-00743-1. Epub 2022 Oct 12. Nat Protoc. 2022. PMID: 36224469 Review.
Cited by
-
Manufacturing the multiscale vascular hierarchy: progress toward solving the grand challenge of tissue engineering.Trends Biotechnol. 2023 Nov;41(11):1400-1416. doi: 10.1016/j.tibtech.2023.04.003. Epub 2023 May 9. Trends Biotechnol. 2023. PMID: 37169690 Free PMC article. Review.
-
Long-term day-by-day tracking of microvascular networks sprouting in fibrin gels: From detailed morphological analyses to general growth rules.APL Bioeng. 2024 Feb 6;8(1):016106. doi: 10.1063/5.0180703. eCollection 2024 Mar. APL Bioeng. 2024. PMID: 38327714 Free PMC article.
-
Developing Porous Fibrin Scaffolds with Tunable Anisotropic Features to Direct Myoblast Orientation.Tissue Eng Part C Methods. 2024 May;30(5):217-228. doi: 10.1089/ten.TEC.2023.0363. Epub 2024 Apr 23. Tissue Eng Part C Methods. 2024. PMID: 38562112 Free PMC article.
-
Clickable PEG-norbornene microgels support suspension bioprinting and microvascular assembly.Acta Biomater. 2025 Jul 1;201:283-296. doi: 10.1016/j.actbio.2025.06.006. Epub 2025 Jun 13. Acta Biomater. 2025. PMID: 40514334
-
Spatially-directed angiogenesis using ultrasound-controlled release of basic fibroblast growth factor from acoustically-responsive scaffolds.Acta Biomater. 2021 Jul 15;129:73-83. doi: 10.1016/j.actbio.2021.04.048. Epub 2021 May 5. Acta Biomater. 2021. PMID: 33964480 Free PMC article.
References
-
- Sanguily J, Martinsen B, Igyarto Z, Pham M. Reducing amputation rates in critical limb ischemia patients via a limb salvage program: a retrospective analysis. Vasc. Dis. Manag. 2016;13:E112–E119.
-
- Yost M. Critical Limb Ischemia Volume I: United States Epidemiology. San Francisco: The SAGE Group; 2010.
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM008353/GM/NIGMS NIH HHS/United States
- R01-HL118259/HL/NHLBI NIH HHS/United States
- R01 HL085339/HL/NHLBI NIH HHS/United States
- T32 HL125242/HL/NHLBI NIH HHS/United States
- T32 DK007378/DK/NIDDK NIH HHS/United States
- T32-GM008353/GM/NIGMS NIH HHS/United States
- T32-DK007378/DK/NIDDK NIH HHS/United States
- T32-DE007057/DE/NIDCR NIH HHS/United States
- T32 DE007057/DE/NIDCR NIH HHS/United States
- R01 HL118259/HL/NHLBI NIH HHS/United States
- T32 GM145304/GM/NIGMS NIH HHS/United States
- T32-HL125242/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

