Evaluation of the neuroprotective effect of taurine in Alzheimer's disease using functional molecular imaging
- PMID: 32968166
- PMCID: PMC7511343
- DOI: 10.1038/s41598-020-72755-4
Evaluation of the neuroprotective effect of taurine in Alzheimer's disease using functional molecular imaging
Abstract
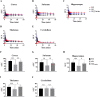
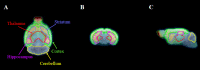
Alzheimer's disease (AD) is a chronic neurodegenerative disorder and the leading cause of dementia, but therapeutic treatment options are limited. Taurine has been reported to have neuroprotective properties against dementia, including AD. The present study aimed to investigate the treatment effect of taurine in AD mice by functional molecular imaging. To elucidate glutamate alterations by taurine, taurine was administered to 5xFAD transgenic mice from 2 months of age, known to apear amyloid deposition. Then, we performed glutamate positron emission tomography (PET) imaging studies for three groups (wild-type, AD, and taurine-treated AD, n = 5 in each group). As a result, brain uptake in the taurine-treated AD group was 31-40% higher than that in the AD group (cortex: 40%, p < 0.05; striatum: 32%, p < 0.01; hippocampus: 36%, p < 0.01; thalamus: 31%, p > 0.05) and 3-14% lower than that in the WT group (cortex: 10%, p > 0.05; striatum: 15%, p > 0.05; hippocampus: 14%, p > 0.05; thalamus: 3%, p > 0.05). However, we did not observe differences in Aβ pathology between the taurine-treated AD and AD groups in immunohistochemistry experiments. Our results reveal that although taurine treatment did not completely recover the glutamate system, it significantly increased metabolic glutamate receptor type 5 brain uptake. Therefore, taurine has therapeutic potential against AD.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Association A. 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018;14:367–429. doi: 10.1016/j.jalz.2018.02.001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

