Hippocampal CA2 sharp-wave ripples reactivate and promote social memory
- PMID: 32968277
- PMCID: PMC7666067
- DOI: 10.1038/s41586-020-2758-y
Hippocampal CA2 sharp-wave ripples reactivate and promote social memory
Abstract
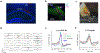
The consolidation of spatial memory depends on the reactivation ('replay') of hippocampal place cells that were active during recent behaviour. Such reactivation is observed during sharp-wave ripples (SWRs)-synchronous oscillatory electrical events that occur during non-rapid-eye-movement (non-REM) sleep1-8 and whose disruption impairs spatial memory3,5,6,8. Although the hippocampus also encodes a wide range of non-spatial forms of declarative memory, it is not yet known whether SWRs are necessary for such memories. Moreover, although SWRs can arise from either the CA3 or the CA2 region of the hippocampus7,9, the relative importance of SWRs from these regions for memory consolidation is unknown. Here we examine the role of SWRs during the consolidation of social memory-the ability of an animal to recognize and remember a member of the same species-focusing on CA2 because of its essential role in social memory10-12. We find that ensembles of CA2 pyramidal neurons that are active during social exploration of previously unknown conspecifics are reactivated during SWRs. Notably, disruption or enhancement of CA2 SWRs suppresses or prolongs social memory, respectively. Thus, SWR-mediated reactivation of hippocampal firing related to recent experience appears to be a general mechanism for binding spatial, temporal and sensory information into high-order memory representations, including social memory.
Conflict of interest statement
Figures




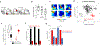

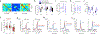






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

