Romiplostim and Eltrombopag in Immune Thrombocytopenia as a Second-Line Treatment
- PMID: 32968581
- PMCID: PMC7505620
- DOI: 10.7759/cureus.9920
Romiplostim and Eltrombopag in Immune Thrombocytopenia as a Second-Line Treatment
Abstract
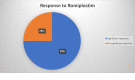
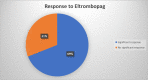
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by platelet count less than 100×109/L and an increased risk of bleeding. The risk of bleeding increases in proportion with the degree of thrombocytopenia. Although several medications are used for primary thrombocytopenia treatment, refractoriness remains a concern. Romiplostim and eltrombopag, two relatively new drugs, have been shown to be successful in ITP treatment after standard treatment failure. The current guidelines recommend their use as a second-line treatment. In this article, we have tried to compare which of these two medications is the best option considering clinical effectiveness, cost-effectiveness, adverse effects, and the possibility of switching between them in case of ineffectiveness. The studies used in this article were found in the PubMed database. All the studies are limited to adults. Based on these studies, both medications seem to be a largely effective, safe option. Romiplostim appears to have slightly fewer adverse effects and higher costs. Switching between thrombopoietin receptor agonists (TRAs) is a successful way to overcome adverse effects and inadequacy according to the currently available literature. We believe that more detailed studies are needed to determine which of these drugs should be considered the first choice, to report long term efficacy and adverse effects, and to determine if treatment guidelines can change regarding the use of TRAs as first-line treatment.
Keywords: eltrombopag; immune thrombocytopenia; itp; platelets; romiplostim; thrombopoietin; thrombopoietin receptor agonists.
Copyright © 2020, Bidika et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Platelet-associated and plasma anti-glycoprotein autoantibodies in chronic ITP. McMillan R, Tani P, Millard F, Berchtold P, Renshaw L, Woods VL. https://pubmed.ncbi.nlm.nih.gov/3651598/ Blood. 1987;70:1040–1045. - PubMed
-
- Long-term clinical outcomes of patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Nørgaard M, Jensen AØ, Engebjerg MC, et al. Blood. 2011;117:3514–3520. - PubMed
-
- Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Rodeghiero F, Stasi R, Gernsheimer T, et al. Blood. 2009;113:2386–2393. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical