A mobile device reducing airborne particulate can improve air quality
- PMID: 32968671
- PMCID: PMC7505793
- DOI: 10.3934/publichealth.2020038
A mobile device reducing airborne particulate can improve air quality
Abstract
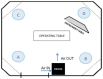
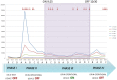
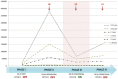
Surgical site infections are the second major cause of hospital acquired infections, accounting for a large part of overall annual medical costs. Airborne particulate is known to be a potential carrier of pathogenic bacteria. We assessed a mobile air particle filter unit for improvement of air quality in an operating room (OR). A new mobile air decontamination and recirculation unit, equipped with a crystalline ultraviolet C (Illuvia® 500 UV) reactor and a HEPA filter, was tested in an OR. Airborne particulate was monitored in four consecutive phases: I) device OFF and OR at rest; II) device OFF and OR in operation; III) device ON and OR in operation; IV) device OFF and OR in operation. We used a particle counter to measure airborne particles of different sizes: ≥0.3, ≥0.5, ≥1, ≥3, ≥5, >10 µm. Activation of the device (phases III) produced a significant reduction (p < 0.05) in airborne particulate of all sizes. Switching the device OFF (phase IV) led to a statistically significant increase (p < 0.05) in the number of particles of most sizes: ≥0.3, ≥0.5, ≥1, ≥3 µm. The device significantly reduced airborne particulate in the OR, improving air quality and possibly lowering the probability of surgical site infections.
Keywords: UV-C; air quality; airborne particulate; healthcare associated infections; operating room; surgical site infections; ultraviolet disinfection.
© 2020 the Author(s), licensee AIMS Press.
Conflict of interest statement
Conflicts of interest: This study was partly funded by Aerobiotix Inc. (Dayton, OH). An Illuvia® 500UV unit was supplied by Aerobiotix Inc for the study. The sponsor was not involved in study design, data collection, analysis or interpretation, the writing of the paper, or in the decision to publish the results. The authors declare no conflict of interest.
Figures
References
-
- European Centre for Disease Prevention and Control. Surveillance of surgical site infections in Europe 2010–2011. Stockholm: ECDC; 2013.
-
- Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. - PubMed
-
- Roy MC, Perl TM. Basics of surgical-site infection surveillance. Infect Control Hosp Epidemiol. 1997;18:659–668. - PubMed
-
- Sadrizadeh S, Pantelic J, Sherman M, et al. Airborne particle dispersion to an operating room environment during sliding and hinged door opening. J Infect Public Health. 2018;11:631–635. - PubMed
-
- Anderson DJ, Kaye KS. Staphylococcal surgical site infections. Infect Dis Clin North Am. 2009;23:53–72. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
