Amino-Supported Palladium Catalyst for Chemo- and Stereoselective Domino Reactions
- PMID: 32969105
- PMCID: PMC7839730
- DOI: 10.1002/anie.202011708
Amino-Supported Palladium Catalyst for Chemo- and Stereoselective Domino Reactions
Abstract
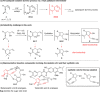
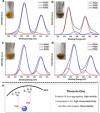
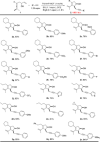
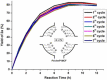
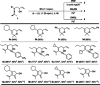
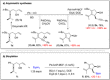
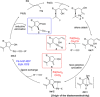
A solid amino-supported palladium catalyst is used in an oxidative domino reaction for the diastereoselective construction of alkyne-substituted cyclopentenol compounds. This heterogeneous catalyst exhibits high efficiency and excellent chemoselectivity, as well as good recyclability. The chemoselectivity of the domino reactions was readily controlled by switching the solvent and catalyst. Asymmetric syntheses and an oxidative carbocyclization-borylation reaction have also been developed based on the heterogeneous palladium catalyst.
Keywords: amines; cyclizations; heterogeneous catalysis; palladium; supported catalysts.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Efficient Heterogeneous Palladium Catalysts in Oxidative Cascade Reactions.Acc Chem Res. 2021 May 4;54(9):2275-2286. doi: 10.1021/acs.accounts.1c00122. Epub 2021 Apr 19. Acc Chem Res. 2021. PMID: 33871980 Free PMC article.
-
Diastereoselective Cyclobutenol Synthesis: A Heterogeneous Palladium-Catalyzed Oxidative Carbocyclization-Borylation of Enallenols.Chemistry. 2019 Jan 2;25(1):210-215. doi: 10.1002/chem.201805118. Epub 2018 Nov 5. Chemistry. 2019. PMID: 30307089
-
Efficient Heterogeneous Palladium-Catalyzed Oxidative Cascade Reactions of Enallenols to Furan and Oxaborole Derivatives.Angew Chem Int Ed Engl. 2020 Jan 27;59(5):1992-1996. doi: 10.1002/anie.201911462. Epub 2019 Dec 13. Angew Chem Int Ed Engl. 2020. PMID: 31729824
-
Recent Development of Palladium-Supported Catalysts for Chemoselective Hydrogenation.Chem Pharm Bull (Tokyo). 2017;65(1):2-9. doi: 10.1248/cpb.c16-00153. Chem Pharm Bull (Tokyo). 2017. PMID: 28049910 Review.
-
Supported Palladium Catalyzed Carbonylative Coupling Reactions using Carbon Monoxide as C1 Source.Chem Rec. 2022 Jan;22(1):e202100157. doi: 10.1002/tcr.202100157. Epub 2021 Aug 21. Chem Rec. 2022. PMID: 34418288 Review.
Cited by
-
Pd Nanoparticles Immobilized on Pyridinic N-Rich Carbon Nanosheets for Promoting Suzuki Cross-Coupling Reactions.Nanomaterials (Basel). 2024 Oct 22;14(21):1690. doi: 10.3390/nano14211690. Nanomaterials (Basel). 2024. PMID: 39513770 Free PMC article.
-
Asymmetric transformation of achiral gold nanoclusters with negative nonlinear dependence between chiroptical activity and enantiomeric excess.Nat Commun. 2023 Jun 22;14(1):3730. doi: 10.1038/s41467-023-39462-w. Nat Commun. 2023. PMID: 37349326 Free PMC article.
-
Efficient Heterogeneous Palladium Catalysts in Oxidative Cascade Reactions.Acc Chem Res. 2021 May 4;54(9):2275-2286. doi: 10.1021/acs.accounts.1c00122. Epub 2021 Apr 19. Acc Chem Res. 2021. PMID: 33871980 Free PMC article.
-
Deciphering the Chameleonic Chemistry of Allenols: Breaking the Taboo of a Onetime Esoteric Functionality.Chem Rev. 2021 Apr 14;121(7):4193-4252. doi: 10.1021/acs.chemrev.0c00986. Epub 2021 Feb 25. Chem Rev. 2021. PMID: 33630581 Free PMC article.
-
Subtilisin integrated artificial plant cell walls as heterogeneous catalysts for asymmetric synthesis of (S)-amides.RSC Adv. 2023 Jul 3;13(29):19975-19980. doi: 10.1039/d3ra02193a. eCollection 2023 Jun 29. RSC Adv. 2023. PMID: 37404321 Free PMC article.
References
-
- Tietze L. F., Brasche G., Gericke K., Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2008.
-
- Tietze L. F., Domino Reactions: Concepts for Efficient Organic Synthesis, Wiley-VCH, Weinheim, 2014.
-
- Nicolaou K. C., Edmonds D. J., Bulger P. G., Angew. Chem. Int. Ed. 2006, 45, 7134–7186; - PubMed
- Angew. Chem. 2006, 118, 7292–7344.
-
- Grondal C., Jeanty M., Enders D., Nat. Chem. 2010, 2, 167–178. - PubMed
-
- Volla C. M. R., Atodiresei I., Rueping M., Chem. Rev. 2014, 114, 2390–2431. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

