Rice stripe virus coat protein induces the accumulation of jasmonic acid, activating plant defence against the virus while also attracting its vector to feed
- PMID: 32969146
- PMCID: PMC7694675
- DOI: 10.1111/mpp.12995
Rice stripe virus coat protein induces the accumulation of jasmonic acid, activating plant defence against the virus while also attracting its vector to feed
Abstract
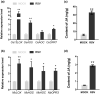
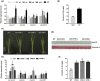
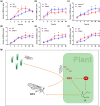
The jasmonic acid (JA) pathway plays crucial roles in plant defence against pathogens and herbivores. Rice stripe virus (RSV) is the type member of the genus Tenuivirus. It is transmitted by the small brown planthopper (SBPH) and causes damaging epidemics in East Asia. The role(s) that JA may play in the tripartite interaction against RSV, its host, and vector are poorly understood. Here, we found that the JA pathway was induced by RSV infection and played a defence role against RSV. The coat protein (CP) was the major viral component responsible for inducing the JA pathway. Methyl jasmonate treatment attracted SBPHs to feed on rice plants while a JA-deficient mutant was less attractive than wild-type rice. SBPHs showed an obvious preference for feeding on transgenic rice lines expressing RSV CP. Our results demonstrate that CP is an inducer of the JA pathway that activates plant defence against RSV while also attracting SBPHs to feed and benefitting viral transmission. This is the first report of the function of JA in the tripartite interaction between RSV, its host, and its vector.
Keywords: Laodelphax striatellus; coat protein; jasmonic acid; rice stripe virus; small brown planthopper (SBPH).
© 2020 The Authors. Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Figures

Similar articles
-
Rice stripe virus-derived siRNAs play different regulatory roles in rice and in the insect vector Laodelphax striatellus.BMC Plant Biol. 2018 Oct 4;18(1):219. doi: 10.1186/s12870-018-1438-7. BMC Plant Biol. 2018. PMID: 30286719 Free PMC article.
-
Rice stripe virus suppresses jasmonic acid-mediated resistance by hijacking brassinosteroid signaling pathway in rice.PLoS Pathog. 2020 Aug 31;16(8):e1008801. doi: 10.1371/journal.ppat.1008801. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32866183 Free PMC article.
-
Rice stripe tenuivirus nonstructural protein 3 hijacks the 26S proteasome of the small brown planthopper via direct interaction with regulatory particle non-ATPase subunit 3.J Virol. 2015 Apr;89(8):4296-310. doi: 10.1128/JVI.03055-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653432 Free PMC article.
-
The small brown planthopper (Laodelphax striatellus) as a vector of the rice stripe virus.Arch Insect Biochem Physiol. 2023 Feb;112(2):e21992. doi: 10.1002/arch.21992. Epub 2022 Dec 27. Arch Insect Biochem Physiol. 2023. PMID: 36575628 Review.
-
Jasmonate biosynthesis and signaling in monocots: a comparative overview.Plant Cell Rep. 2013 Jun;32(6):815-27. doi: 10.1007/s00299-013-1400-y. Epub 2013 Mar 2. Plant Cell Rep. 2013. PMID: 23455708 Review.
Cited by
-
Rice stripe virus p2 protein interacts with ATG5 and is targeted for degradation by autophagy.Front Microbiol. 2023 Apr 28;14:1191403. doi: 10.3389/fmicb.2023.1191403. eCollection 2023. Front Microbiol. 2023. PMID: 37187544 Free PMC article.
-
The dynamics of N6-methyladenine RNA modification in interactions between rice and plant viruses.Genome Biol. 2021 Jun 24;22(1):189. doi: 10.1186/s13059-021-02410-2. Genome Biol. 2021. PMID: 34167554 Free PMC article.
-
Coat protein of rice stripe virus enhances autophagy activity through interaction with cytosolic glyceraldehyde-3-phosphate dehydrogenases, a negative regulator of plant autophagy.Stress Biol. 2023 Mar 23;3(1):3. doi: 10.1007/s44154-023-00084-3. Stress Biol. 2023. PMID: 37676568 Free PMC article.
-
Molecular Hydrogen Confers Resistance to Rice Stripe Virus.Microbiol Spectr. 2023 Feb 22;11(2):e0441722. doi: 10.1128/spectrum.04417-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36840556 Free PMC article.
-
Advancing the Rose Rosette Virus Minireplicon and Encapsidation System by Incorporating GFP, Mutations, and the CMV 2b Silencing Suppressor.Viruses. 2022 Apr 17;14(4):836. doi: 10.3390/v14040836. Viruses. 2022. PMID: 35458566 Free PMC article.
References
-
- Boughton, A.J. , Hoover, K. and Felton, G.W. (2006) Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae . Entomologia Experimentalis et Applicata, 120, 175–188.
-
- Cheng, J. , Zhao, W. , Lou, Y. and Zhu, Z. (2001) Intra‐ and inter‐specific effects of the brown planthopper and white backed planthopper on their population performance. Journal of Asia‐Pacific Entomology, 4, 85–92.
-
- Csorba, T. , Kontra, L. and Burgyan, J. (2015) Viral silencing suppressors: tools forged to fine‐tune host–pathogen coexistence. Virology, 479–480, 85–103. - PubMed
-
- Dar, T.A. , Uddin, M. , Khan, M.M.A. , Hakeem, K.R. and Jaleel, H. (2015) Jasmonates counter plant stress: a review. Environmental and Experimental Botany, 115, 49–57.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

