Safety and Efficacy of Elexacaftor/Tezacaftor/Ivacaftor for 24 Weeks or Longer in People with Cystic Fibrosis and One or More F508del Alleles: Interim Results of an Open-Label Phase 3 Clinical Trial
- PMID: 32969708
- PMCID: PMC8020728
- DOI: 10.1164/rccm.202008-3176LE
Safety and Efficacy of Elexacaftor/Tezacaftor/Ivacaftor for 24 Weeks or Longer in People with Cystic Fibrosis and One or More F508del Alleles: Interim Results of an Open-Label Phase 3 Clinical Trial
Figures
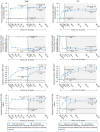
References
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. - PubMed
-
- Cystic Fibrosis Trust UK Cystic Fibrosis Registry 2018 Annual Data Report London, United Kingdom: Cystic Fibrosis Trust; 2019 [accessed 2020 Dec 10]. Available from: https://www.cysticfibrosis.org.uk/the-work-we-do/uk-cf-registry/reportin...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

