Liver enzyme CYP2D6 gene and tardive dyskinesia
- PMID: 32969762
- PMCID: PMC7586356
- DOI: 10.2217/pgs-2020-0065
Liver enzyme CYP2D6 gene and tardive dyskinesia
Abstract
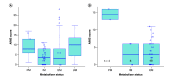
Background: Tardive dyskinesia (TD) is an iatrogenic involuntary movement disorder occurring after extended antipsychotic use with unclear pathogenesis. CYP2D6 is a liver enzyme involved in antipsychotic metabolism and a well-studied gene candidate for TD. Materials & methods: We tested predicted CYP2D6 metabolizer phenotype with TD occurrence and severity in our two samples of European chronic schizophrenia patients (total n = 198, of which 82 had TD). Results: TD occurrence were associated with extreme metabolizer phenotype, controlling for age and sex (p = 0.012). In other words, individuals with either increased and no CYP2D6 activity were at higher risk of having TD. Conclusion: Unlike most previous findings, TD occurrence may be associated with both extremes of CYP2D6 metabolic activity rather than solely for poor metabolizers.
Keywords: CYP2D6; metabolizer phenotype; pharmacogenetics; schizophrenia; tardive dyskinesia.
Conflict of interest statement
The authors thank the Ministry of Research and Innovation of Ontario, for funding the IMPACT project. CC Zai, AK Tiwari, DJ DJ Müller and JL Kennedy have been supported by the Genome Canada Genomic Applications Partnership Program (GAPP) and the CAMH Foundation. DJ Müller was supported by the Canadian Institutes of Health Research (CIHR operating grant MOP 142192), the National Institutes of Health (R01MH085801), the CAMH Foundation (J Murphy Professorship) and received a Brain & Behaviour Research (NARSAD) Independent Investigator Award, the M Smith New Investigator Salary Prize for Research in Schizophrenia (CIHR) and an Early Researcher Award by the Ministry of Research and Innovation of Ontario. G Remington was supported by the Canadian Institutes of Health Research (CIHR), as well as the Research Hospital Fund – Canadian Foundation for Innovation (RHF-CFI). CC Zai, AK Tiwari, DJ Müller and JL Kennedy are investigators in two pharmacogenetic studies where genetic test kits were provided as in-kind contribution by Assurex Health (Myriad Neuroscience) to evaluate the feasibility of pharmacogenetic testing in clinical practice and potential benefits of pharmacogenetic testing compared with treatment as usual. They have not received any payments or received any equity, stocks or options from this company or any other pharmacogenetic companies. CC Zai, AK Tiwari, DJ Müller and JL Kennedy are authors in two filed genetic patents assessing risk for antipsychotic-induced weight gain. JL Kennedy has received honoraria from Novartis, Roche and Eli Lilly corporations, and is an unpaid member of the scientific advisory board of AssureRx Corp. HY Meltzer has received grants or is or was a consultant to: Abbott Labs, ACADIA, Alkemes, Bristol Myers Squibb, DaiNippon Sumitomo, Eli Lilly, EnVivo, Janssen, Otsuka, Pfizer, Roche, Sunovion and BiolineRx. HY Meltzer is a shareholder of ACADIA and Glaxo Smith Kline. In the past three years JA Lieberman reports having received research funding or is a member of the advisory board of Allon, Alkermes, Bioline, GlaxoSmithKline Intracellular Therapies, Lilly, Merck, Novartis, Pfizer, Pierre Fabre, Psychogenics, F Hoffmann-La Roche LTD, Sepracor (Sunovion) and Targacept. JA Lieberman receives no direct financial compensation or salary support for participation in these research, consulting or advisory board activities. SG Potkin: consultancy/board of advisors/honoraria: American Psychiatric Association, Astra Zeneca, Bristol-Myers Squibb, Cortex, Dainippon-Sumitomo, Janssen Pharmaceutica, Novartis, Otsuka, Pfizer, Roche, Schering Plough, Vanda; research grants: Amgen, Bristol-Myers Squibb, Dainippon Sumitomo, Elan, En Vivo, Forest Laboratories, Janssen Pharmaceutica, Merck, Novartis, Otsuka, Pfizer, Solvay Pharmaceuticals, Roche, Sunovion, NIH, Harvard Massachusetts General Hospital, Brigham and Women’s Hospital, Vanda, speakers’ bureau: Lundbeck, Otsuka, ISCTM, Novartis, Pfizer, Sunovion. In the past 3, years G Remington has received consultant fees from HLS Therapeutics and Mitsubishi Tanabe Pharma Corporation, as well as research support from HLS Therapeutics. He holds no commercial investments in any pharmaceutical company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
References
-
- van Harten PN, Tenback DE. Tardive dyskinesia: clinical presentation and treatment. Int. Rev. Neurobiol. 98, 187–210 (2011). - PubMed
-
- Solmi M, Pigato G, Kane JM, Correll CU. Clinical risk factors for the development of tardive dyskinesia. J. Neurol. Sci. 389, 21–27 (2018). - PubMed
-
- Carbon M, Hsieh CH, Kane JM, Correll CU. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J. Clin. Psychiat. 78(3), e264–e278 (2017). - PubMed
-
- Teo JT, Edwards MJ, Bhatia K. Tardive dyskinesia is caused by maladaptive synaptic plasticity: a hypothesis. Mov. Disord. 27(10), 1205–1215 (2012). - PubMed
