Rapid Determination of SARS-CoV-2 antibodies using a bedside, point-of-Care, serological test
- PMID: 32969769
- PMCID: PMC7580567
- DOI: 10.1080/22221751.2020.1826892
Rapid Determination of SARS-CoV-2 antibodies using a bedside, point-of-Care, serological test
Abstract
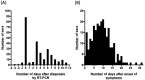
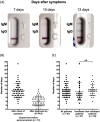
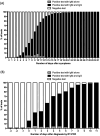
Background: Several serological tests for SARS-CoV-2 have been developed or use, but most have only been validated on few samples, and none provide medical practitioners with an easy-to-use, self-contained, bedside test with high accuracy. Material and methods: Two-hundred fifty-six sera from 101 patients hospitalized with SARS-CoV-2 infection (positive RT-PCR) and 50 control sera were tested for IgM/IgG using the NG-Test IgM-IgG COVID all-in-one assay. The seroconversion dynamic was assessed by symptom onset and day of RT-PCR diagnosis. Results: Among the SARS-CoV-2 infected patients, positive IgG and/or IgM result was observed for 67.3% of patients (68/101), including 17 (16.8%) already positive at the day of RT-PCR, and 51 (50.5%) with observable seroconversion, and 32.7% (33/101) remained negative as subsequent sampling was not possible (patient discharge or death). The sensitivity increased with the delay between onset of symptoms and sampling, going from 29.1%, 78.2% and 86.5% for the time periods of 0-9-, 10-14- and >14-days after the onset of symptoms, respectively. Cumulative sensitivity, specificity, Positive Predictive Value and Negative Predictive Value were 97.0%, 100%, 100% and 96.2%, respectively 15-days after the onset of symptoms. No difference in seroconversion delay was observed regardless of whether patients received ventilation. Conclusions: The NG-test is a bedside serological assay that could serve as a complementary source of diagnostic information to RT-PCR and chest imaging. It may also be useful to monitor immunological status of medical and non-medical workers during the ongoing pandemic, and the general population after social distancing measures have eased.
Keywords: COVID-19; Diagnostics; bedside; diagnosis; rapid test; serology.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- WHO . Novel coronavirus – China. [cited 2020 Jan 19] Available from: http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ .
-
- World Health Organization (WHO) . Outbreak Investigation. [cited 2020 Mar 13] Available from: https://www.who.int/hac/techguidance/training/outbreak%20investigation_e....
-
- WHO . Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19), Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mis... (2020).
-
- Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) (July 3rd, 2020). Available from: https://coronavirus.jhu.edu/map.html.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
