Revisiting dithiadiaza macrocyclic chelators for copper-64 PET imaging
- PMID: 32970072
- PMCID: PMC7967274
- DOI: 10.1039/d0dt02787a
Revisiting dithiadiaza macrocyclic chelators for copper-64 PET imaging
Abstract
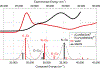
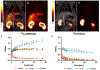
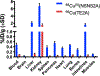
Synthesis and characterisation of a dithiadiaza chelator NSNS2A, as well as copper complexes thereof are reported in this paper. Solution structures of copper(i/ii) complexes were calculated using density functional theory (DFT) and validated by both NMR and EPR spectroscopy. DFT calculations revealed a switch in the orientation of tetragonal distortion upon protonation, which might be responsible for poor stability of the Cu(II)NSNS2A complex in aqueous media, whilst the same switch in tetragonal distortion was experimentally observed by changing the solvent. The chelator was radiolabeled with 64Cu and evaluated using PET/MRI in rats. Despite a favorable redox potential to stabilize the cuprous state in vivo, the 64Cu(II)NSNS2A complex showed suboptimal stability compared to its tetraazamacrocyclic analogue, 64Cu(TE2A), with a significant 64Cu uptake in the liver.
Figures

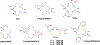

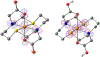


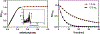


Similar articles
-
Coordination Chemistry of Bifunctional Chemical Agents Designed for Applications in 64Cu PET Imaging for Alzheimer's Disease.Inorg Chem. 2017 Nov 20;56(22):13801-13814. doi: 10.1021/acs.inorgchem.7b01883. Epub 2017 Nov 7. Inorg Chem. 2017. PMID: 29112419 Free PMC article.
-
Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes.J Med Chem. 2004 Mar 11;47(6):1465-74. doi: 10.1021/jm030383m. J Med Chem. 2004. PMID: 14998334
-
Macrocyclic diamide ligand systems: potential chelators for 64Cu- and 68Ga-based positron emission tomography imaging agents.Inorg Chem. 2009 Aug 3;48(15):7117-26. doi: 10.1021/ic900307f. Inorg Chem. 2009. PMID: 19588930 Free PMC article.
-
Chelators for copper radionuclides in positron emission tomography radiopharmaceuticals.J Labelled Comp Radiopharm. 2014 Apr;57(4):224-30. doi: 10.1002/jlcr.3165. Epub 2013 Dec 18. J Labelled Comp Radiopharm. 2014. PMID: 24347474 Free PMC article. Review.
-
Cross-bridged macrocyclic chelators for stable complexation of copper radionuclides for PET imaging.Q J Nucl Med Mol Imaging. 2008 Jun;52(2):185-92. Epub 2007 Nov 28. Q J Nucl Med Mol Imaging. 2008. PMID: 18043536 Free PMC article. Review.
Cited by
-
Copper Coordination Chemistry of Sulfur Pendant Cyclen Derivatives: An Attempt to Hinder the Reductive-Induced Demetalation in 64/67Cu Radiopharmaceuticals.Inorg Chem. 2021 Aug 2;60(15):11530-11547. doi: 10.1021/acs.inorgchem.1c01550. Epub 2021 Jul 19. Inorg Chem. 2021. PMID: 34279088 Free PMC article.
-
Chelation of Theranostic Copper Radioisotopes with S-Rich Macrocycles: From Radiolabelling of Copper-64 to In Vivo Investigation.Molecules. 2022 Jun 28;27(13):4158. doi: 10.3390/molecules27134158. Molecules. 2022. PMID: 35807404 Free PMC article.
-
Is Smaller Better? Cu2+/Cu+ Coordination Chemistry and Copper-64 Radiochemical Investigation of a 1,4,7-Triazacyclononane-Based Sulfur-Rich Chelator.Inorg Chem. 2023 Dec 18;62(50):20621-20633. doi: 10.1021/acs.inorgchem.3c00621. Epub 2023 Apr 28. Inorg Chem. 2023. PMID: 37115633 Free PMC article.
References
-
- Bass LA, Wang M, Welch MJ and Anderson CJ, Bioconjug Chem, 2000, 11, 527–532. - PubMed
-
- Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL and Anderson CJ, J Med Chem, 2004, 47, 1465–1474. - PubMed
-
- Zia NA, Cullinane C, Van Zuylekom JK, Waldeck K, McInnes LE, Buncic G, Haskali MB, Roselt PD, Hicks RJ and Donnelly PS, Angew Chem Int Ed Engl, 2019, 58, 14991–14994. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

