Revisiting dithiadiaza macrocyclic chelators for copper-64 PET imaging
- PMID: 32970072
- PMCID: PMC7967274
- DOI: 10.1039/d0dt02787a
Revisiting dithiadiaza macrocyclic chelators for copper-64 PET imaging
Abstract
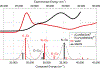
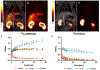
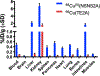
Synthesis and characterisation of a dithiadiaza chelator NSNS2A, as well as copper complexes thereof are reported in this paper. Solution structures of copper(i/ii) complexes were calculated using density functional theory (DFT) and validated by both NMR and EPR spectroscopy. DFT calculations revealed a switch in the orientation of tetragonal distortion upon protonation, which might be responsible for poor stability of the Cu(II)NSNS2A complex in aqueous media, whilst the same switch in tetragonal distortion was experimentally observed by changing the solvent. The chelator was radiolabeled with 64Cu and evaluated using PET/MRI in rats. Despite a favorable redox potential to stabilize the cuprous state in vivo, the 64Cu(II)NSNS2A complex showed suboptimal stability compared to its tetraazamacrocyclic analogue, 64Cu(TE2A), with a significant 64Cu uptake in the liver.
Figures

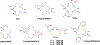

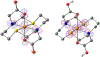


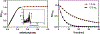


References
-
- Bass LA, Wang M, Welch MJ and Anderson CJ, Bioconjug Chem, 2000, 11, 527–532. - PubMed
-
- Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL and Anderson CJ, J Med Chem, 2004, 47, 1465–1474. - PubMed
-
- Zia NA, Cullinane C, Van Zuylekom JK, Waldeck K, McInnes LE, Buncic G, Haskali MB, Roselt PD, Hicks RJ and Donnelly PS, Angew Chem Int Ed Engl, 2019, 58, 14991–14994. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

