The evaluation of 1-tetralone and 4-chromanone derivatives as inhibitors of monoamine oxidase
- PMID: 32970293
- PMCID: PMC7512223
- DOI: 10.1007/s11030-020-10143-w
The evaluation of 1-tetralone and 4-chromanone derivatives as inhibitors of monoamine oxidase
Abstract
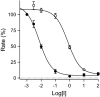
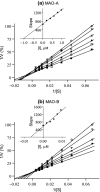
Monoamine oxidase (MAO) is of much clinical relevance, and inhibitors of this enzyme are used in the treatment for neuropsychiatric and neurodegenerative disorders such as depression and Parkinson's disease. The present study synthesises and evaluates the MAO inhibition properties of a series of 33 1-tetralone and 4-chromanone derivatives in an attempt to discover high-potency compounds and to expand on the structure-activity relationships of MAO inhibition by these classes. Among these series, eight submicromolar MAO-A inhibitors and 28 submicromolar MAO-B inhibitors are reported, with all compounds acting as specific inhibitors of the MAO-B isoform. The most potent inhibitor was a 1-tetralone derivative (1h) with IC50 values of 0.036 and 0.0011 µM for MAO-A and MAO-B, respectively. Interestingly, with the reduction of 1-tetralones to the corresponding alcohols, a decrease in MAO inhibition potency is observed. Among these 1-tetralol derivatives, 1p (IC50 = 0.785 μM) and 1o (IC50 = 0.0075 μM) were identified as particularly potent inhibitors of MAO-A and MAO-B, respectively. Potent compounds such as those reported here may act as leads for the future development of MAO-B specific inhibitors. The present study describes the MAO inhibitory activities of a series of 1-tetralone and 4-chromanone derivatives. Numerous high-potency MAO-B specific inhibitors were identified.
Keywords: 1-Tetralone; 4-Chromanone; Inhibition; Monoamine oxidase; Parkinson’s disease.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

