CD73 contributes to anti-inflammatory properties of afferent lymphatic endothelial cells in humans and mice
- PMID: 32970335
- PMCID: PMC7821194
- DOI: 10.1002/eji.201948432
CD73 contributes to anti-inflammatory properties of afferent lymphatic endothelial cells in humans and mice
Abstract
CD73 is an important ectoenzyme responsible for the production of extracellular adenosine. It is involved in regulating inflammatory responses and cell migration and is overexpressed in various cancers. The functions of CD73 in blood endothelial cells are understood in detail, but its role on afferent lymphatics remains unknown. Moreover, anti-CD73 antibodies are now used in multiple clinical cancer trials, but their effects on different endothelial cell types have not been studied. This study reveals that a previously unknown role of CD73 on afferent lymphatics is to dampen immune responses. Knocking it out or suppressing it by siRNA leads to the upregulation of inflammation-associated genes on lymphatic endothelial cells and a more pro-inflammatory phenotype of interacting dendritic cells in vitro and in vivo. In striking contrast, anti-CD73 antibodies had only negligible effects on the gene expression of lymphatic- and blood-endothelial cells. Our data thus reveal new functions of lymphatic CD73 and indicate a low likelihood of endothelial cell-related adverse effects by CD73 targeting therapeutic antibodies.
Keywords: CD73; dendritic cells; lymphatic endothelial cells; siRNA; vascular biology.
© 2020 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
S. J. and M. S. own stocks of Faron Pharmaceuticals. The other authors have no additional commercial and financial interests.
Figures



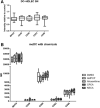
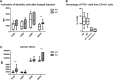

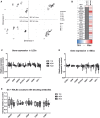
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

