Resistance to Some But Not Other Dimeric Lindenane Sesquiterpenoid Esters Is Mediated by Mutations in a Plasmodium falciparum Esterase
- PMID: 32970404
- PMCID: PMC11075783
- DOI: 10.1021/acsinfecdis.0c00487
Resistance to Some But Not Other Dimeric Lindenane Sesquiterpenoid Esters Is Mediated by Mutations in a Plasmodium falciparum Esterase
Abstract
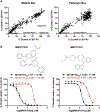
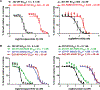
Unique lindenane sesquiterpenoid dimers from Chloranthecae spp. were recently identified with promising in vitro antiplasmodial activity and potentially novel mechanisms of action. To gain mechanistic insights to this new class of natural products, in vitro selection of Plasmodium falciparum resistance to the most active antiplasmodial compound, chlorajaponilide C, was explored. In all selected resistant clones, the half-maximal effective concentration (EC50) of chlorajaponilide C increased >250-fold, and whole genome sequencing revealed mutations in the recently discovered P. falciparum prodrug activation and resistance esterase (PfPARE). Chlorajaponilide C was highly potent (mean EC50 = 1.6 nM, n = 34) against fresh Ugandan P. falciparum isolates. The analysis of the structure-resistance relationships revealed that in vitro potency of a subset of lindenane sesquiterpenoid dimers was not mediated by PfPARE mutations. Thus, chlorajaponilide C, but not some related compounds, required parasite esterase activity for in vitro potency, and those compounds serve as the foundation for development of potent and selective antimalarials.
Keywords: Malaria Box; Pathogen Box; PfPARE; Plasmodium; lindenane sesquiterpenoid dimers; structure-resistance relationship study.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
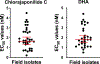

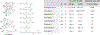
Similar articles
-
Plasmodium falciparum Resistance to a Lead Benzoxaborole Due to Blocked Compound Activation and Altered Ubiquitination or Sumoylation.mBio. 2020 Jan 28;11(1):e02640-19. doi: 10.1128/mBio.02640-19. mBio. 2020. PMID: 31992618 Free PMC article.
-
Esterase mutation is a mechanism of resistance to antimalarial compounds.Nat Commun. 2017 Jan 20;8:14240. doi: 10.1038/ncomms14240. Nat Commun. 2017. PMID: 28106035 Free PMC article.
-
Nanomolar Antimalarial Agents against Chloroquine-Resistant Plasmodium falciparum from Medicinal Plants and Their Structure-Activity Relationships.J Nat Prod. 2017 Jan 27;80(1):96-107. doi: 10.1021/acs.jnatprod.6b00744. Epub 2016 Dec 20. J Nat Prod. 2017. PMID: 27997206 Free PMC article.
-
Lindenane sesquiterpenoid monomers and oligomers: Chemistry and pharmacological activities.Phytochemistry. 2023 Nov;215:113866. doi: 10.1016/j.phytochem.2023.113866. Epub 2023 Sep 20. Phytochemistry. 2023. PMID: 37739202 Review.
-
Hybrid molecules with potential in vitro antiplasmodial and in vivo antimalarial activity against drug-resistant Plasmodium falciparum.Med Res Rev. 2020 May;40(3):931-971. doi: 10.1002/med.21643. Epub 2019 Nov 6. Med Res Rev. 2020. PMID: 31692025 Review.
Cited by
-
Prodrug activation in malaria parasites mediated by an imported erythrocyte esterase, acylpeptide hydrolase (APEH).Proc Natl Acad Sci U S A. 2025 Mar 11;122(10):e2417682122. doi: 10.1073/pnas.2417682122. Epub 2025 Mar 4. Proc Natl Acad Sci U S A. 2025. PMID: 40035755
-
Babesia BdFE1 esterase is required for the anti-parasitic activity of the ACE inhibitor fosinopril.J Biol Chem. 2023 Nov;299(11):105313. doi: 10.1016/j.jbc.2023.105313. Epub 2023 Oct 4. J Biol Chem. 2023. PMID: 37797695 Free PMC article.
-
Prodrug activation in malaria parasites mediated by an imported erythrocyte esterase, acylpeptide hydrolase (APEH).bioRxiv [Preprint]. 2024 Aug 31:2024.08.30.610542. doi: 10.1101/2024.08.30.610542. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Mar 11;122(10):e2417682122. doi: 10.1073/pnas.2417682122. PMID: 39257815 Free PMC article. Updated. Preprint.
-
Transcriptome-Based Identification of a Functional Fasciola hepatica Carboxylesterase B.Pathogens. 2021 Nov 10;10(11):1454. doi: 10.3390/pathogens10111454. Pathogens. 2021. PMID: 34832612 Free PMC article.
-
A Single Nucleotide Polymorphism Translates into a Radical Amino Acid Substitution at the Ligand-Binding Site in Fasciola hepatica Carboxylesterase B.Genes (Basel). 2022 Oct 19;13(10):1899. doi: 10.3390/genes13101899. Genes (Basel). 2022. PMID: 36292784 Free PMC article.
References
-
- WHO (2019-12-04) World Malaria Report 2019, World Health Organization, Geneva.
-
- Laurens MB (2018) The promise of a malaria vaccine: are we closer? Annu. Rev. Microbiol. 72, 273–292. - PubMed
-
- Wells TNC, Hooft van Huijsduijnen R, and Van Voorhis WC (2015) Malaria medicines: a glass half full? Nat. Rev. Drug Discovery 14, 424–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

