CMV viral load kinetics as surrogate endpoints after allogeneic transplantation
- PMID: 32970635
- PMCID: PMC7773411
- DOI: 10.1172/JCI133960
CMV viral load kinetics as surrogate endpoints after allogeneic transplantation
Abstract
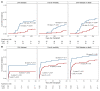
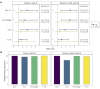
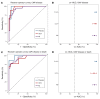
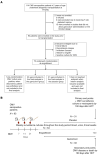
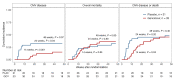
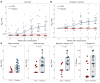
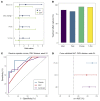
BACKGROUNDViral load (VL) surrogate endpoints transformed development of HIV and hepatitis C therapeutics. Surrogate endpoints for CMV-related morbidity and mortality could advance development of antiviral treatments. Although observational data support using CMV VL as a trial endpoint, randomized controlled trials (RCTs) demonstrating direct associations between virological markers and clinical endpoints are lacking.METHODSWe performed CMV DNA PCR on frozen serum samples from the only placebo-controlled RCT of ganciclovir for early treatment of CMV after hematopoietic cell transplantation (HCT). We used established criteria to assess VL kinetics as surrogates for CMV disease or death by weeks 8, 24, and 48 after randomization and quantified antiviral effects captured by each marker. We used ensemble-based machine learning to assess the predictive ability of VL kinetics and performed this analysis on a ganciclovir prophylaxis RCT for validation.RESULTSVL suppression with ganciclovir reduced cumulative incidence of CMV disease and death for 20 years after HCT. Mean VL, peak VL, and change in VL during the first 5 weeks of treatment fulfilled the Prentice definition for surrogacy, capturing more than 95% of ganciclovir's effect, and yielded highly sensitive and specific predictions by week 48. In the prophylaxis trial, the viral shedding rate satisfied the Prentice definition for CMV disease by week 24.CONCLUSIONSOur results support using CMV VL kinetics as surrogates for CMV disease, provide a framework for developing CMV preventative and therapeutic agents, and support reductions in VL as the mechanism through which antivirals reduce CMV disease.FUNDINGMerck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
Keywords: Clinical Trials; Drug therapy; Infectious disease; Stem cell transplantation.
Conflict of interest statement
Figures


References
-
- Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002;185(3):273–282. doi: 10.1086/338624. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

