Characteristics and Maternal and Birth Outcomes of Hospitalized Pregnant Women with Laboratory-Confirmed COVID-19 - COVID-NET, 13 States, March 1-August 22, 2020
- PMID: 32970655
- PMCID: PMC7727497
- DOI: 10.15585/mmwr.mm6938e1
Characteristics and Maternal and Birth Outcomes of Hospitalized Pregnant Women with Laboratory-Confirmed COVID-19 - COVID-NET, 13 States, March 1-August 22, 2020
Abstract
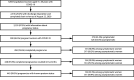
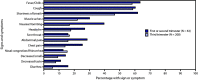
Pregnant women might be at increased risk for severe coronavirus disease 2019 (COVID-19) (1,2). The COVID-19-Associated Hospitalization Surveillance Network (COVID-NET) (3) collects data on hospitalized pregnant women with laboratory-confirmed SARS-CoV-2, the virus that causes COVID-19; to date, such data have been limited. During March 1-August 22, 2020, approximately one in four hospitalized women aged 15-49 years with COVID-19 was pregnant. Among 598 hospitalized pregnant women with COVID-19, 54.5% were asymptomatic at admission. Among 272 pregnant women with COVID-19 who were symptomatic at hospital admission, 16.2% were admitted to an intensive care unit (ICU), and 8.5% required invasive mechanical ventilation. During COVID-19-associated hospitalizations, 448 of 458 (97.8%) completed pregnancies resulted in a live birth and 10 (2.2%) resulted in a pregnancy loss. Testing policies based on the presence of symptoms might miss COVID-19 infections during pregnancy. Surveillance of pregnant women with COVID-19, including those with asymptomatic infections, is important to understand the short- and long-term consequences of COVID-19 for mothers and newborns. Identifying COVID-19 in women during birth hospitalizations is important to guide preventive measures to protect pregnant women, parents, newborns, other patients, and hospital personnel. Pregnant women and health care providers should be made aware of the potential risks for severe COVID-19 illness, adverse pregnancy outcomes, and ways to prevent infection.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Evan J. Anderson reports grants from MedImmune, Regeneron, PaxVax, Pfizer, GSK, Merck, Novavax, Sanofi-Pasteur, Micron, and Janssen, and personal fees from AbbVie, Pfizer, Sanofi Pasteur and Kentucky BioProcessing, Inc. outside the submitted work. William Schaffner reports personal fees from VBI Vaccines, outside the submitted work. No other potential conflicts of interest were disclosed.
Figures
Comment in
-
An imperative to offer pregnant and lactating women access to the COVID-19 vaccination roll-out programme.S Afr Med J. 2021 Apr 12;111(6):567-569. S Afr Med J. 2021. PMID: 34382568
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

