Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings
- PMID: 32970782
- PMCID: PMC7514033
- DOI: 10.1371/journal.ppat.1008817
Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings
Abstract
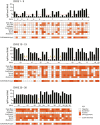
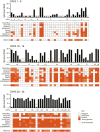
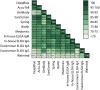
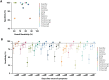
There is a clear requirement for an accurate SARS-CoV-2 antibody test, both as a complement to existing diagnostic capabilities and for determining community seroprevalence. We therefore evaluated the performance of a variety of antibody testing technologies and their potential use as diagnostic tools. Highly specific in-house ELISAs were developed for the detection of anti-spike (S), -receptor binding domain (RBD) and -nucleocapsid (N) antibodies and used for the cross-comparison of ten commercial serological assays-a chemiluminescence-based platform, two ELISAs and seven colloidal gold lateral flow immunoassays (LFIAs)-on an identical panel of 110 SARS-CoV-2-positive samples and 50 pre-pandemic negatives. There was a wide variation in the performance of the different platforms, with specificity ranging from 82% to 100%, and overall sensitivity from 60.9% to 87.3%. However, the head-to-head comparison of multiple sero-diagnostic assays on identical sample sets revealed that performance is highly dependent on the time of sampling, with sensitivities of over 95% seen in several tests when assessing samples from more than 20 days post onset of symptoms. Furthermore, these analyses identified clear outlying samples that were negative in all tests, but were later shown to be from individuals with mildest disease presentation. Rigorous comparison of antibody testing platforms will inform the deployment of point-of-care technologies in healthcare settings and their use in the monitoring of SARS-CoV-2 infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- NHS. Guidance and Standard Operating Procedure COVID-19 Virus Testing in NHS Laboratories (https://www.rcpath.org/uploads/assets/90111431-8aca-4614-b06633d07e2a3dd...)
-
- Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19 (https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specim...)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

