Spatiotemporal restriction of endothelial cell calcium signaling is required during leukocyte transmigration
- PMID: 32970800
- PMCID: PMC7953625
- DOI: 10.1084/jem.20192378
Spatiotemporal restriction of endothelial cell calcium signaling is required during leukocyte transmigration
Abstract
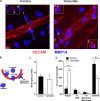
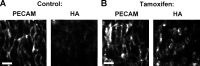
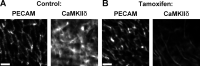
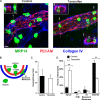
Endothelial cell calcium flux is critical for leukocyte transendothelial migration (TEM), which in turn is essential for the inflammatory response. Intravital microscopy of endothelial cell calcium dynamics reveals that calcium increases locally and transiently around the transmigration pore during TEM. Endothelial calmodulin (CaM), a key calcium signaling protein, interacts with the IQ domain of IQGAP1, which is localized to endothelial junctions and is required for TEM. In the presence of calcium, CaM binds endothelial calcium/calmodulin kinase IIδ (CaMKIIδ). Disrupting the function of CaM or CaMKII with small-molecule inhibitors, expression of a CaMKII inhibitory peptide, or expression of dominant negative CaMKIIδ significantly reduces TEM by interfering with the delivery of the lateral border recycling compartment (LBRC) to the site of TEM. Endothelial CaMKII is also required for TEM in vivo as shown in two independent mouse models. These findings highlight novel roles for endothelial CaM and CaMKIIδ in transducing the spatiotemporally restricted calcium signaling required for TEM.
© 2020 Dalal et al.
Conflict of interest statement
Disclosures: I.M. Grumbach reported grants from the National Institutes of Health, the Department of Veterans Affairs, and the American Heart Association outside the submitted work. No other disclosures were reported.
Figures



References
-
- Akerboom, J., Rivera J.D., Guilbe M.M., Malavé E.C., Hernandez H.H., Tian L., Hires S.A., Marvin J.S., Looger L.L., and Schreiter E.R.. 2009. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J. Biol. Chem. 284:6455–6464. 10.1074/jbc.M807657200 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

