The GM-CSF-IRF5 signaling axis in eosinophils promotes antitumor immunity through activation of type 1 T cell responses
- PMID: 32970801
- PMCID: PMC7953737
- DOI: 10.1084/jem.20190706
The GM-CSF-IRF5 signaling axis in eosinophils promotes antitumor immunity through activation of type 1 T cell responses
Abstract
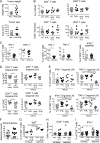
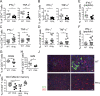
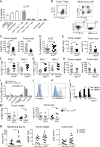
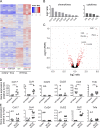
The depletion of eosinophils represents an efficient strategy to alleviate allergic asthma, but the consequences of prolonged eosinophil deficiency for human health remain poorly understood. We show here that the ablation of eosinophils severely compromises antitumor immunity in syngeneic and genetic models of colorectal cancer (CRC), which can be attributed to defective Th1 and CD8+ T cell responses. The specific loss of GM-CSF signaling or IRF5 expression in the eosinophil compartment phenocopies the loss of the entire lineage. GM-CSF activates IRF5 in vitro and in vivo and can be administered recombinantly to improve tumor immunity. IL-10 counterregulates IRF5 activation by GM-CSF. CRC patients whose tumors are infiltrated by large numbers of eosinophils also exhibit robust CD8 T cell infiltrates and have a better prognosis than patients with eosinophillow tumors. The combined results demonstrate a critical role of eosinophils in tumor control in CRC and introduce the GM-CSF-IRF5 axis as a critical driver of the antitumor activities of this versatile cell type.
© 2020 Arnold et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures








Similar articles
-
Interferon-gamma enhances human eosinophil effector functions induced by granulocyte-macrophage colony-stimulating factor or interleukin-5.Immunol Lett. 2008 Jun 15;118(1):88-95. doi: 10.1016/j.imlet.2008.03.005. Epub 2008 Apr 10. Immunol Lett. 2008. PMID: 18440651
-
Peripheral blood CD4 but not CD8 t-lymphocytes in patients with exacerbation of asthma transcribe and translate messenger RNA encoding cytokines which prolong eosinophil survival in the context of a Th2-type pattern: effect of glucocorticoid therapy.Am J Respir Cell Mol Biol. 1995 May;12(5):567-78. doi: 10.1165/ajrcmb.12.5.7742019. Am J Respir Cell Mol Biol. 1995. PMID: 7742019
-
Immunization with a syngeneic tumor infected with recombinant vaccinia virus expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) induces tumor regression and long-lasting systemic immunity.J Immunother. 1997 Nov;20(6):449-59. doi: 10.1097/00002371-199711000-00004. J Immunother. 1997. PMID: 9409450
-
[The assessment of Eo-CSF and cytokines as eosinopoietic factor].Nihon Rinsho. 1993 Mar;51(3):736-40. Nihon Rinsho. 1993. PMID: 8492450 Review. Japanese.
-
[Eosinophil apoptosis and asthma].Pol Merkur Lekarski. 2004 Jul;17(97):73-5. Pol Merkur Lekarski. 2004. PMID: 15559618 Review. Polish.
Cited by
-
Construction and Validation of Pyroptosis-Related lncRNA Prediction Model for Colon Adenocarcinoma and Immune Infiltration Analysis.Dis Markers. 2022 Sep 17;2022:4492608. doi: 10.1155/2022/4492608. eCollection 2022. Dis Markers. 2022. PMID: 36168326 Free PMC article.
-
Circulating white blood cell traits and colorectal cancer risk: A Mendelian randomisation study.Int J Cancer. 2024 Jan 1;154(1):94-103. doi: 10.1002/ijc.34691. Epub 2023 Aug 14. Int J Cancer. 2024. PMID: 37578112 Free PMC article.
-
Deep learning-based quantification of eosinophils and lymphocytes shows complementary prognostic effects in colorectal cancer patients.NPJ Precis Oncol. 2025 Jun 13;9(1):175. doi: 10.1038/s41698-025-00955-0. NPJ Precis Oncol. 2025. PMID: 40514395 Free PMC article.
-
Heterogeneity of Intestinal Tissue Eosinophils: Potential Considerations for Next-Generation Eosinophil-Targeting Strategies.Cells. 2021 Feb 17;10(2):426. doi: 10.3390/cells10020426. Cells. 2021. PMID: 33671475 Free PMC article. Review.
-
A risk signature based on endoplasmic reticulum stress-associated genes predicts prognosis and immunity in pancreatic cancer.Front Mol Biosci. 2023 Nov 29;10:1298077. doi: 10.3389/fmolb.2023.1298077. eCollection 2023. Front Mol Biosci. 2023. PMID: 38106991 Free PMC article.
References
-
- American Joint Committee on Cancer . 2002. AJCC Cancer Staging Manual. Sixth edition. Springer, New York.
-
- Arnold, I.C., Artola-Borán M., Tallón de Lara P., Kyburz A., Taube C., Ottemann K., van den Broek M., Yousefi S., Simon H.U., and Müller A.. 2018. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J. Exp. Med. 215:2055–2072. 10.1084/jem.20172049 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

