Viral infection and smell loss: The case of COVID-19
- PMID: 32970861
- PMCID: PMC7537178
- DOI: 10.1111/jnc.15197
Viral infection and smell loss: The case of COVID-19
Abstract
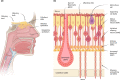
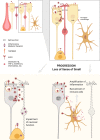
Olfactory disorders have been increasingly reported in individuals infected with SARS-CoV-2, the virus causing the coronavirus disease 2019 (COVID-19). Losing the sense of smell has a strong impact on the quality of life, since it may lead to malnutrition, weight loss, food poisoning, depression, and exposure to dangerous chemicals. Individuals who suffer from anosmia (inability to smell) also cannot sense the flavor of food, which is a combination of taste and smell. Interestingly, infected individuals have reported sudden loss of smell with no congested nose, as is frequently observed in common colds or other upper respiratory tract infections. These observations suggest that SARS-CoV-2 infection leads to olfactory loss through a distinct mechanism, which is still unclear. This article provides an overview of olfactory loss and the recent findings relating to COVID-19. Possible mechanisms of SARS-CoV-2-induced olfactory loss are also discussed.
Keywords: SARS-CoV-2; anosmia; coronavirus; olfaction; olfactory sensory neuron; smell loss.
© 2020 International Society for Neurochemistry.
Figures
References
-
- Adney, D. R. , van Doremalen, N. , Brown, V. R. , Bushmaker, T. , Scott, D. , de Wit, E. , Bowen, R. A. , & Munster, V. J. (2014). Replication and shedding of MERS‐CoV in upper respiratory tract of inoculated dromedary camels. Emerging Infectious Diseases, 20, 1999–2005. 10.3201/eid2012.141280 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- 2013/07937-8/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 2016/24471-0/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 2018/18633-3/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 2019/06982-6/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 2019/26767-2/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

