BEHAVIORAL AND NEUROBIOLOGICAL MECHANISMS OF PAVLOVIAN AND INSTRUMENTAL EXTINCTION LEARNING
- PMID: 32970967
- PMCID: PMC8428921
- DOI: 10.1152/physrev.00016.2020
BEHAVIORAL AND NEUROBIOLOGICAL MECHANISMS OF PAVLOVIAN AND INSTRUMENTAL EXTINCTION LEARNING
Abstract
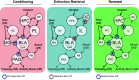
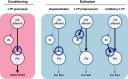
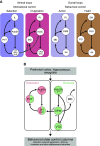
This article reviews the behavioral neuroscience of extinction, the phenomenon in which a behavior that has been acquired through Pavlovian or instrumental (operant) learning decreases in strength when the outcome that reinforced it is removed. Behavioral research indicates that neither Pavlovian nor operant extinction depends substantially on erasure of the original learning but instead depends on new inhibitory learning that is primarily expressed in the context in which it is learned, as exemplified by the renewal effect. Although the nature of the inhibition may differ in Pavlovian and operant extinction, in either case the decline in responding may depend on both generalization decrement and the correction of prediction error. At the neural level, Pavlovian extinction requires a tripartite neural circuit involving the amygdala, prefrontal cortex, and hippocampus. Synaptic plasticity in the amygdala is essential for extinction learning, and prefrontal cortical inhibition of amygdala neurons encoding fear memories is involved in extinction retrieval. Hippocampal-prefrontal circuits mediate fear relapse phenomena, including renewal. Instrumental extinction involves distinct ensembles in corticostriatal, striatopallidal, and striatohypothalamic circuits as well as their thalamic returns for inhibitory (extinction) and excitatory (renewal and other relapse phenomena) control over operant responding. The field has made significant progress in recent decades, although a fully integrated biobehavioral understanding still awaits.
Keywords: Pavlovian conditioning; amygdala; extinction; hippocampus; instrumental conditioning; prefrontal cortex; striatum.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Bouton ME. Extinction: behavioral mechanisms and their implications. In: Learning Theory and Behavior, Vol 1 of Learning and Memory: a Comprehensive Reference, edited byMenzel R.Oxford: Academic Press, 2017, p. 61–83.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

