Macrophages Maintain Epithelium Integrity by Limiting Fungal Product Absorption
- PMID: 32970988
- PMCID: PMC7646275
- DOI: 10.1016/j.cell.2020.08.048
Macrophages Maintain Epithelium Integrity by Limiting Fungal Product Absorption
Abstract
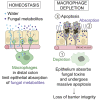
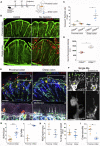
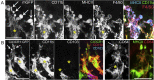
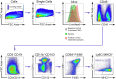
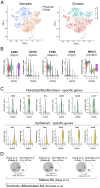
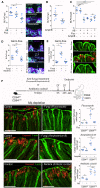
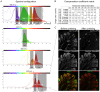
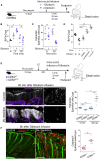
The colon is primarily responsible for absorbing fluids. It contains a large number of microorganisms including fungi, which are enriched in its distal segment. The colonic mucosa must therefore tightly regulate fluid influx to control absorption of fungal metabolites, which can be toxic to epithelial cells and lead to barrier dysfunction. How this is achieved remains unknown. Here, we describe a mechanism by which the innate immune system allows rapid quality check of absorbed fluids to avoid intoxication of colonocytes. This mechanism relies on a population of distal colon macrophages that are equipped with "balloon-like" protrusions (BLPs) inserted in the epithelium, which sample absorbed fluids. In the absence of macrophages or BLPs, epithelial cells keep absorbing fluids containing fungal products, leading to their death and subsequent loss of epithelial barrier integrity. These results reveal an unexpected and essential role of macrophages in the maintenance of colon-microbiota interactions in homeostasis. VIDEO ABSTRACT.
Keywords: colon; epithelium integrity; fluid absorption; fungi; gut; macrophages; metabolites; microbiota; protrusions; toxins.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





Comment in
-
Colonic Macrophages Combat Fungal Intoxication: Metchnikoff Would Be Pleased.Cell. 2020 Oct 15;183(2):305-307. doi: 10.1016/j.cell.2020.09.046. Cell. 2020. PMID: 33064986
-
Water quality check: macrophages setting the standards.Cell Res. 2021 Jan;31(1):3-4. doi: 10.1038/s41422-020-00429-x. Cell Res. 2021. PMID: 33139927 Free PMC article. No abstract available.
-
Guardians of the epithelium: macrophages protect against toxic fungal derivatives.Mucosal Immunol. 2021 May;14(3):542-543. doi: 10.1038/s41385-020-00369-0. Epub 2021 Jan 25. Mucosal Immunol. 2021. PMID: 33495494 Free PMC article.
References
-
- Al-Bahrani A.Z., Darwish A., Hamza N., Benson J., Eddleston J.M., Snider R.H., Nylén E.S., Becker K.L., Barclay G.R., Ammori B.J. Gut barrier dysfunction in critically ill surgical patients with abdominal compartment syndrome. Pancreas. 2010;39:1064–1069. - PubMed
-
- Bain C.C., Scott C.L., Uronen-Hansson H., Gudjonsson S., Jansson O., Grip O., Guilliams M., Malissen B., Agace W.W., Mowat A.M.I. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. - PMC - PubMed
-
- Ballard S.T., Hunter J.H., Taylor A.E. Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium. Annu. Rev. Nutr. 1995;15:35–55. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

