SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2
- PMID: 32970989
- PMCID: PMC7489987
- DOI: 10.1016/j.cell.2020.09.033
SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2
Abstract
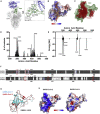
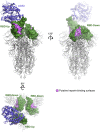
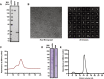
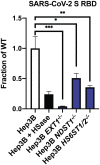
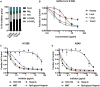
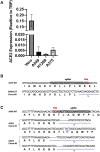
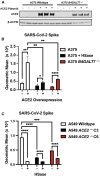
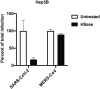
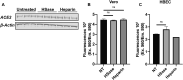
We show that SARS-CoV-2 spike protein interacts with both cellular heparan sulfate and angiotensin-converting enzyme 2 (ACE2) through its receptor-binding domain (RBD). Docking studies suggest a heparin/heparan sulfate-binding site adjacent to the ACE2-binding site. Both ACE2 and heparin can bind independently to spike protein in vitro, and a ternary complex can be generated using heparin as a scaffold. Electron micrographs of spike protein suggests that heparin enhances the open conformation of the RBD that binds ACE2. On cells, spike protein binding depends on both heparan sulfate and ACE2. Unfractionated heparin, non-anticoagulant heparin, heparin lyases, and lung heparan sulfate potently block spike protein binding and/or infection by pseudotyped virus and authentic SARS-CoV-2 virus. We suggest a model in which viral attachment and infection involves heparan sulfate-dependent enhancement of binding to ACE2. Manipulation of heparan sulfate or inhibition of viral adhesion by exogenous heparin presents new therapeutic opportunities.
Keywords: COVID-19; SARS-CoV-2; coronavirus; heparan sulfate; heparan sulfate-binding proteins; heparin; lung epithelial cells; pseudotyped virus; spike proteins.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests J.D.E. is a co-founder of TEGA Therapeutics. J.D.E. and the Regents of the University of California have licensed a University invention to and have an equity interest in TEGA Therapeutics. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. C.A.G. and B.E.T. are employees of TEGA Therapeutics.
Figures




Update of
-
SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2.bioRxiv [Preprint]. 2020 Jul 14:2020.07.14.201616. doi: 10.1101/2020.07.14.201616. bioRxiv. 2020. Update in: Cell. 2020 Nov 12;183(4):1043-1057.e15. doi: 10.1016/j.cell.2020.09.033. PMID: 32699853 Free PMC article. Updated. Preprint.
Comment in
-
Engaging the spikes: heparan sulfate facilitates SARS-CoV-2 spike protein binding to ACE2 and potentiates viral infection.Signal Transduct Target Ther. 2021 Jan 29;6(1):39. doi: 10.1038/s41392-021-00470-1. Signal Transduct Target Ther. 2021. PMID: 33514685 Free PMC article. No abstract available.
References
-
- Bouwman K.M., Delpont M., Broszeit F., Berger R., Weerts E.A.W.S., Lucas M.N., Delverdier M., Belkasmi S., Papanikolaou A., Boons G.J., et al. Guinea Fowl Coronavirus Diversity Has Phenotypic Consequences for Glycan and Tissue Binding. J. Virol. 2019;93 doi: 10.1128/JVI.00067-19. - DOI - PMC - PubMed
-
- Casu B., Guerrini M., Guglieri S., Naggi A., Perez M., Torri G., Cassinelli G., Ribatti D., Carminati P., Giannini G., et al. Undersulfated and glycol-split heparins endowed with antiangiogenic activity. J. Med. Chem. 2004;47:838–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

