Targeting HDAC3 in the DBA/2J spontaneous mouse model of glaucoma
- PMID: 32971093
- PMCID: PMC8344090
- DOI: 10.1016/j.exer.2020.108244
Targeting HDAC3 in the DBA/2J spontaneous mouse model of glaucoma
Abstract
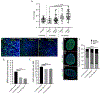
High intraocular pressure (IOP) is the most common risk factor associated with glaucoma in humans. While lowering IOP is effective at reducing the rate of retinal ganglion cell (RGC) loss, to date, no treatment exists to directly preserve these cells affected by damage to the optic nerve. Recently, histone deacetylase-3 (HDAC3) has become a potential therapeutic target because it plays an important role in the early nuclear atrophic events that precede RGC death. Conditional knockout or inhibition of HDAC3 prevents histone deacetylation, heterochromatin formation, apoptosis, and eventual RGC loss following acute optic nerve injury. Using these approaches to repress HDAC3 activity, we tested whether targeting HDAC3 protects RGCs from ganglion cell-specific BRN3A expression loss, total somatic cell loss, and optic nerve degeneration in the DBA/2J mouse model of spontaneous glaucoma. Targeted ablation of Hdac3 activity did not protect RGCs from axonal degeneration or somatic cell death in the DBA/2J mouse model of glaucoma. However, inhibition of HDAC3 activity using RGFP966 conferred mild protection against somatic cell loss in the ganglion cell layer in aged DBA/2J mice. Further experimentation is necessary to determine whether other class I HDACs may serve as potential therapeutic targets in chronic models of glaucoma.
Keywords: Epigenetics; Glaucoma; HDAC3; Optic nerve; RGFP966; Retinal ganglion cell.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

References
-
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW, 2002. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet 30, 81–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

