Prevention of Perinatal HIV Transmission in an Area of High HIV Prevalence in the United States
- PMID: 32971142
- PMCID: PMC7752838
- DOI: 10.1016/j.jpeds.2020.09.041
Prevention of Perinatal HIV Transmission in an Area of High HIV Prevalence in the United States
Abstract
Objective: To evaluate the uptake of perinatal HIV preventive interventions by the risk of perinatal HIV transmission in mother-infant pairs in a high-HIV prevalence area in the US.
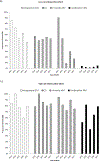
Study design: This was a retrospective cohort study of mother-infant pairs with perinatal HIV exposure during 2013-2017 managed at a subspecialty pediatric HIV program in Washington, DC. We collected demographic data, maternal HIV history, delivery mode, maternal and infant antiretroviral drug (ARV) use, and infant HIV test results. We compared the uptake of recommended preventive interventions in low-risk (ie, mothers on antiretroviral therapy [ART] with viral suppression) and high-risk (mothers without ART or viral suppression) mother-infant pairs using the Pearson chi-square, Fisher exact, and Wilcoxon rank-sum tests and logistic regression.
Results: We analyzed 551 HIV-exposed infants (HEIs) and 542 mothers living with HIV. The majority of mothers received ARVs (95.5%), had HIV RNA ≤1000 copies/mL before delivery (81.9%), and received intrapartum zidovudine (ZDV; 65.5%). The majority of all HEIs were low risk (82.6%) and received postpartum ARVs (98.9%). Among the low-risk infants, 53.2% were delivered via cesarean delivery (CD), and 62.9% and 96.5% were administered intrapartum and postpartum ZDV, respectively. Among high-risk infants, 84.4% were delivered via CD, 78.1% received intrapartum ZDV, and 62.5% received combination ART. Nine high-risk infants acquired HIV perinatally.
Conclusion: In an area of high HIV prevalence in the US, a large proportion of low-risk HEIs received intrapartum ZDV and were delivered via CD. We also observed missed opportunities for the prevention of perinatal HIV transmission.
Keywords: antiretroviral treatment; cesarean delivery; infants; mothers; pregnancy.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Centers for Disease Control and Prevention. Revised Recommendations for HIV Screening of Pregnant Women: Perinatal Counseling and Guidelines Consultation. MMWR Weekly. 2001;50:59–86. - PubMed
-
- Centers for Disease Control and Prevention. Enhanced perinatal surveillance - 15 areas, 2005–2008 HIV Surveillance Supplemental Report 2011; 16(2). http://www.cdc.gov/hiv/topics/surveillance/resources/reports Accessed [11/6/2018].
-
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

