Glutathionylated and Fe-S cluster containing hMIA40 (CHCHD4) regulates ROS and mitochondrial complex III and IV activities of the electron transport chain
- PMID: 32971361
- PMCID: PMC7511737
- DOI: 10.1016/j.redox.2020.101725
Glutathionylated and Fe-S cluster containing hMIA40 (CHCHD4) regulates ROS and mitochondrial complex III and IV activities of the electron transport chain
Abstract
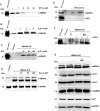
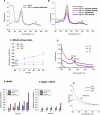
Human MIA40, an intermembrane space (IMS) import receptor of mitochondria harbors twin CX9C motifs for stability while its CPC motif is known to facilitate the import of IMS bound proteins. Site-directed mutagenesis complemented by MALDI on in vivo hMIA40 protein shows that a portion of MIA40 undergoes reversible S-glutathionylation at three cysteines in the twin CX9C motifs and the lone cysteine 4 residue. We find that HEK293T cells expressing hMIA40 mutant defective for glutathionylation are compromised in the activities of complexes III and IV of the Electron Transport Chain (ETC) and enhance Reactive Oxygen Species (ROS) levels. Immunocapture studies show MIA40 interacting with complex III. Interestingly, glutathionylated MIA40 can transfer electrons to cytochrome C directly. However, Fe-S clusters associated with the CPC motif are essential to facilitate the two-electron to one-electron transfer for reducing cytochrome C. These results suggest that hMIA40 undergoes glutathionylation to maintain ROS levels and for optimum function of complexes III and IV of ETC. Our studies shed light on a novel post-translational modification of hMIA40 and its ability to act as a redox switch to regulate the ETC and cellular redox homeostasis.
Keywords: Complex III and IV; Electron transport chain; Fe–S clusters; Glutathionylation; MIA40 (CHCHD4); Reactive oxygen species.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures






Similar articles
-
Human mitochondrial MIA40 (CHCHD4) is a component of the Fe-S cluster export machinery.Biochem J. 2015 Oct 15;471(2):231-41. doi: 10.1042/BJ20150012. Epub 2015 Aug 14. Biochem J. 2015. PMID: 26275620
-
Structural and functional roles of the conserved cysteine residues of the redox-regulated import receptor Mia40 in the intermembrane space of mitochondria.J Biol Chem. 2009 Jan 16;284(3):1353-63. doi: 10.1074/jbc.M805035200. Epub 2008 Nov 14. J Biol Chem. 2009. PMID: 19011240
-
AIFM1 is a component of the mitochondrial disulfide relay that drives complex I assembly through efficient import of NDUFS5.EMBO J. 2022 Sep 1;41(17):e110784. doi: 10.15252/embj.2022110784. Epub 2022 Jul 20. EMBO J. 2022. PMID: 35859387 Free PMC article.
-
AIF meets the CHCHD4/Mia40-dependent mitochondrial import pathway.Biochim Biophys Acta Mol Basis Dis. 2020 Jun 1;1866(6):165746. doi: 10.1016/j.bbadis.2020.165746. Epub 2020 Feb 24. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32105825 Review.
-
Regulation of COX Assembly and Function by Twin CX9C Proteins-Implications for Human Disease.Cells. 2021 Jan 20;10(2):197. doi: 10.3390/cells10020197. Cells. 2021. PMID: 33498264 Free PMC article. Review.
Cited by
-
Mitochondrial dysfunction induced by bedaquiline as an anti-Toxoplasma alternative.Vet Res. 2023 Dec 19;54(1):123. doi: 10.1186/s13567-023-01252-z. Vet Res. 2023. PMID: 38115043 Free PMC article.
-
COVID-19-induced neurological symptoms: focus on the role of metal ions.Inflammopharmacology. 2023 Apr;31(2):611-631. doi: 10.1007/s10787-023-01176-2. Epub 2023 Mar 9. Inflammopharmacology. 2023. PMID: 36892679 Free PMC article. Review.
-
S-Glutathionylation and S-Nitrosylation in Mitochondria: Focus on Homeostasis and Neurodegenerative Diseases.Int J Mol Sci. 2022 Dec 13;23(24):15849. doi: 10.3390/ijms232415849. Int J Mol Sci. 2022. PMID: 36555492 Free PMC article. Review.
-
CHCHD4 Oxidoreductase Activity: A Comprehensive Analysis of the Molecular, Functional, and Structural Properties of Its Redox-Regulated Substrates.Molecules. 2025 May 10;30(10):2117. doi: 10.3390/molecules30102117. Molecules. 2025. PMID: 40430290 Free PMC article. Review.
-
Piceatannol Induces Mitochondrial Dysfunction in Toxoplasma gondii.Microorganisms. 2025 May 25;13(6):1203. doi: 10.3390/microorganisms13061203. Microorganisms. 2025. PMID: 40572091 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

