Glutaredoxin deficiency promotes activation of the transforming growth factor beta pathway in airway epithelial cells, in association with fibrotic airway remodeling
- PMID: 32971362
- PMCID: PMC7509797
- DOI: 10.1016/j.redox.2020.101720
Glutaredoxin deficiency promotes activation of the transforming growth factor beta pathway in airway epithelial cells, in association with fibrotic airway remodeling
Abstract
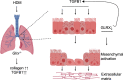
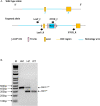
S-glutathionylation of reactive protein cysteines is a post-translational event that plays a critical role in transducing signals from oxidants into biological responses. S-glutathionylation can be reversed by the deglutathionylating enzyme glutaredoxin (GLRX). We have previously demonstrated that ablation of Glrx sensitizes mice to the development of parenchymal lung fibrosis(1). It remains unclear whether GLRX also controls airway fibrosis, a clinical feature relevant to asthma and chronic obstructive pulmonary disease, and whether GLRX controls the biology of airway epithelial cells, which have been implicated in the pathophysiology of these diseases. In the present study we utilized a house dust mite (HDM) model of allergic airway disease in wild type (WT) and Glrx-/- mice on a C57BL/6 background prone to develop airway fibrosis, and tracheal basal stem cells derived from WT mice, global Glrx-/- mice, or bi-transgenic mice allowing conditional ablation of the Glrx gene. Herein we show that absence of Glrx led to enhanced HDM-induced collagen deposition, elevated levels of transforming growth factor beta 1 (TGFB1) in the bronchoalveolar lavage, and resulted in increases in airway hyperresponsiveness. Airway epithelial cells isolated from Glrx-/- mice or following conditional ablation of Glrx showed spontaneous increases in secretion of TGFB1. Glrx-/- basal cells also showed spontaneous TGFB pathway activation, in association with increased expression of mesenchymal genes, including collagen 1a1 and fibronectin. Overall, these findings suggest that GLRX regulates airway fibrosis via a mechanism(s) that involve the plasticity of basal cells, the stem cells of the airways.
Keywords: Asthma; Basal cells; COPD; Fibrosis; S-glutathionylation.
Published by Elsevier B.V.
Conflict of interest statement
Yvonne Janssen-Heininger and Vikas Anathy hold patents: United States Patent No. 8,679,811, “Treatments Involving Glutaredoxins and Similar Agents” (YJ-H, VA), United States Patent No. 8,877,447, “Detection of Glutathionylated Proteins” (YJ-H), United States Patent, 9,907,828, “Treatments of oxidative stress conditions” (YJ-H, VA).
Yvonne Janssen-Heininger and Vikas Anathy have received consulting fees from Celdara Medical LLC for their contributions to the proposed commercialization of glutaredoxin for the treatment of pulmonary fibrosis.
Figures







Similar articles
-
Reducing protein oxidation reverses lung fibrosis.Nat Med. 2018 Aug;24(8):1128-1135. doi: 10.1038/s41591-018-0090-y. Epub 2018 Jul 9. Nat Med. 2018. PMID: 29988126 Free PMC article.
-
Endoplasmic Reticulum Oxidative Stress Promotes Glutathione-Dependent Oxidation of Collagen-1A1 and Promotes Lung Fibroblast Activation.Am J Respir Cell Mol Biol. 2024 Nov;71(5):589-602. doi: 10.1165/rcmb.2023-0379OC. Am J Respir Cell Mol Biol. 2024. PMID: 39042020 Free PMC article.
-
Absence of c-Jun NH2-terminal kinase 1 protects against house dust mite-induced pulmonary remodeling but not airway hyperresponsiveness and inflammation.Am J Physiol Lung Cell Mol Physiol. 2014 May 1;306(9):L866-75. doi: 10.1152/ajplung.00153.2013. Epub 2014 Mar 7. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24610935 Free PMC article.
-
Epithelial NF-κB orchestrates house dust mite-induced airway inflammation, hyperresponsiveness, and fibrotic remodeling.J Immunol. 2013 Dec 15;191(12):5811-21. doi: 10.4049/jimmunol.1301329. Epub 2013 Nov 13. J Immunol. 2013. PMID: 24227776 Free PMC article.
-
Redox Regulation via Glutaredoxin-1 and Protein S-Glutathionylation.Antioxid Redox Signal. 2020 Apr 1;32(10):677-700. doi: 10.1089/ars.2019.7963. Epub 2020 Jan 23. Antioxid Redox Signal. 2020. PMID: 31813265 Free PMC article. Review.
Cited by
-
Post-translational modifications of collagen and its related diseases in metabolic pathways.Acta Pharm Sin B. 2025 Apr;15(4):1773-1795. doi: 10.1016/j.apsb.2025.02.007. Epub 2025 Feb 11. Acta Pharm Sin B. 2025. PMID: 40486838 Free PMC article. Review.
-
EEF1A1 transcription cofactor gene polymorphism is associated with muscle gene expression and residual feed intake in Nelore cattle.Mamm Genome. 2022 Dec;33(4):619-628. doi: 10.1007/s00335-022-09959-8. Epub 2022 Jul 11. Mamm Genome. 2022. PMID: 35816191
-
Dehydroglutathione, a glutathione derivative to introduce non-reversible glutathionylation.RSC Chem Biol. 2025 May 21;6(7):1156-1164. doi: 10.1039/d5cb00052a. eCollection 2025 Jul 2. RSC Chem Biol. 2025. PMID: 40487857 Free PMC article.
-
Recent evidence from omic analysis for redox signalling and mitochondrial oxidative stress in COPD.J Inflamm (Lond). 2022 Jul 11;19(1):10. doi: 10.1186/s12950-022-00308-9. J Inflamm (Lond). 2022. PMID: 35820851 Free PMC article. Review.
-
Emerging chemistry and biology in protein glutathionylation.Curr Opin Chem Biol. 2022 Dec;71:102221. doi: 10.1016/j.cbpa.2022.102221. Epub 2022 Oct 9. Curr Opin Chem Biol. 2022. PMID: 36223700 Free PMC article. Review.
References
-
- Anathy V., Lahue K.G., Chapman D.G., Chia S.B., Casey D.T., Aboushousha R., van der Velden J.L.J., Elko E., Hoffman S.M., McMillan D.H., Jones J.T., Nolin J.D., Abdalla S., Schneider R., Seward D.J., Roberson E.C., Liptak M.D., Cousins M.E., Butnor K.J., Taatjes D.J., Budd R.C., Irvin C.G., Ho Y.S., Hakem R., Brown K.K., Matsui R., Bachschmid M.M., Gomez J.L., Kaminski N., van der Vliet A., Janssen-Heininger Y.M.W. Reducing protein oxidation reverses lung fibrosis. Nat. Med. 2018;24(8):1128–1135. doi: 10.1038/s41591-018-0090-y. Epub 2018/07/11, PubMed PMID: 29988126; PMCID: PMC6204256. - DOI - PMC - PubMed
-
- Chakir J., Shannon J., Molet S., Fukakusa M., Elias J., Laviolette M., Boulet L.P., Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J. Allergy Clin. Immunol. 2003;111(6):1293–1298. doi: 10.1067/mai.2003.1557. Epub 2003/06/06, PubMed PMID: 12789232. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

