Oxidoreductases in Glycoprotein Glycosylation, Folding, and ERAD
- PMID: 32971745
- PMCID: PMC7563561
- DOI: 10.3390/cells9092138
Oxidoreductases in Glycoprotein Glycosylation, Folding, and ERAD
Abstract
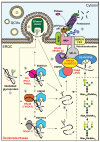
N-linked glycosylation and sugar chain processing, as well as disulfide bond formation, are among the most common post-translational protein modifications taking place in the endoplasmic reticulum (ER). They are essential modifications that are required for membrane and secretory proteins to achieve their correct folding and native structure. Several oxidoreductases responsible for disulfide bond formation, isomerization, and reduction have been shown to form stable, functional complexes with enzymes and chaperones that are involved in the initial addition of an N-glycan and in folding and quality control of the glycoproteins. Some of these oxidoreductases are selenoproteins. Recent studies also implicate glycan machinery-oxidoreductase complexes in the recognition and processing of misfolded glycoproteins and their reduction and targeting to ER-associated degradation. This review focuses on the intriguing cooperation between the glycoprotein-specific cell machineries and ER oxidoreductases, and highlights open questions regarding the functions of many members of this large family.
Keywords: ER quality control; ERAD; PDI; calnexin; mannosidase; oligosaccharyltransferase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gross E., Sevier C.S., Heldman N., Vitu E., Bentzur M., Kaiser C.A., Thorpe C., Fass D. Generating disulfides enzymatically: Reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc. Natl. Acad. Sci. USA. 2006;103:299–304. doi: 10.1073/pnas.0506448103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

