Quantitative Analysis of Somatostatin and Dopamine Receptors Gene Expression Levels in Non-functioning Pituitary Tumors and Association with Clinical and Molecular Aggressiveness Features
- PMID: 32971845
- PMCID: PMC7565399
- DOI: 10.3390/jcm9093052
Quantitative Analysis of Somatostatin and Dopamine Receptors Gene Expression Levels in Non-functioning Pituitary Tumors and Association with Clinical and Molecular Aggressiveness Features
Abstract
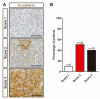
The primary treatment for non-functioning pituitary tumors (NFPTs) is surgery, but it is often unsuccessful. Previous studies have reported that NFPTs express receptors for somatostatin (SST1-5) and dopamine (DRDs) providing a rationale for the use of dopamine agonists and somatostatin analogues. Here, we systematically assessed SST1-5 and DRDs expression by real-time quantitative PCR (RT-qPCR) in a large group of patients with NFPTs (n = 113) and analyzed their potential association with clinical and molecular aggressiveness features. SST1-5 expression was also evaluated by immunohistochemistry. SST3 was the predominant SST subtype detected, followed by SST2, SST5, and SST1. DRD2 was the dominant DRD subtype, followed by DRD4, DRD5, and DRD1. A substantial proportion of NFPTs displayed marked expression of SST2 and SST5. No major association between SSTs and DRDs expression and clinical and molecular aggressiveness features was observed in NFPTs.
Keywords: dopamine receptor; invasion; non-functioning pituitary tumors; pituitary tumor; somatostatin receptor.
Conflict of interest statement
This work was partly supported by a grant from Novartis Oncology Spain to A.S.-M. and D.A.C. Novartis Oncology did not have any role in the design, data collection and analysis of the study. The authors confirm that there are no other conflicts of interest regarding this study.
Figures


References
Grants and funding
- PI16/00175 to A.S-M. and D.A.C; PI16/00264 to R.M.L./ISCIII-Subdirección General de Evaluación y Fomento de la Investigación co-funded with Fondos FEDER
- A-0006-2017 and A-0055-2018 to A.S-M, C-0015-2014 and RC-0006-2018 to D.A.C./Sistema Andaluz de Salud
- BFU2016-80360-R to J.P.C.; PID2019-105564RB-I00 to R.M.L/Ministerio de Ciencia, Innovación
- BIO-0139 to J.P.C and R.M.L/Junta de Andalucía
- NFPAMOL/Novartis Oncology Spain

