A Novel Cis-Acting RNA Structural Element Embedded in the Core Coding Region of the Hepatitis C Virus Genome Directs Internal Translation Initiation of the Overlapping Core+1 ORF
- PMID: 32972019
- PMCID: PMC7554737
- DOI: 10.3390/ijms21186974
A Novel Cis-Acting RNA Structural Element Embedded in the Core Coding Region of the Hepatitis C Virus Genome Directs Internal Translation Initiation of the Overlapping Core+1 ORF
Abstract
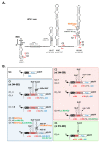
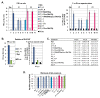
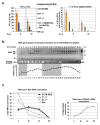
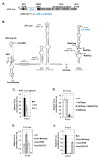
Hepatitis C virus (HCV) genome translation is initiated via an internal ribosome entry site (IRES) embedded in the 5'-untranslated region (5'UTR). We have earlier shown that the conserved RNA stem-loops (SL) SL47 and SL87 of the HCV core-encoding region are important for viral genome translation in cell culture and in vivo. Moreover, we have reported that an open reading frame overlapping the core gene in the +1 frame (core+1 ORF) encodes alternative translation products, including a protein initiated at the internal AUG codons 85/87 of this frame (nt 597-599 and 603-605), downstream of SL87, which is designated core+1/Short (core+1/S). Here, we provide evidence for SL47 and SL87 possessing a novel cis-acting element that directs the internal translation initiation of core+1/S. Firstly, using a bicistronic dual luciferase reporter system and RNA-transfection experiments, we found that nucleotides 344-596 of the HCV genotype-1a and -2a genomes support translation initiation at the core+1 frame AUG codons 85/87, when present in the sense but not the opposite orientation. Secondly, site-directed mutagenesis combined with an analysis of ribosome-HCV RNA association elucidated that SL47 and SL87 are essential for this alternative translation mechanism. Finally, experiments using cells transfected with JFH1 replicons or infected with virus-like particles showed that core+1/S expression is independent from the 5'UTR IRES and does not utilize the polyprotein initiation codon, but it requires intact SL47 and SL87 structures. Thus, SL47 and SL87, apart from their role in viral polyprotein translation, are necessary elements for mediating the internal translation initiation of the alternative core+1/S ORF.
Keywords: RNA translation; core+1 ORF; hepatitis C virus; stem-loops.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Wilder J., Patel K. A Review of the Natural History of Chronic Hepatitis C Infection. N. Am. J. Med. Sci. 2014;7:1–7. doi: 10.7156/najms.2014.0701001. - DOI
-
- World Health Organization Hepatitis C. [(accessed on 31 July 2020)]; Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

