Drug Repositioning: New Approaches and Future Prospects for Life-Debilitating Diseases and the COVID-19 Pandemic Outbreak
- PMID: 32972027
- PMCID: PMC7551028
- DOI: 10.3390/v12091058
Drug Repositioning: New Approaches and Future Prospects for Life-Debilitating Diseases and the COVID-19 Pandemic Outbreak
Abstract
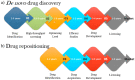
Traditionally, drug discovery utilises a de novo design approach, which requires high cost and many years of drug development before it reaches the market. Novel drug development does not always account for orphan diseases, which have low demand and hence low-profit margins for drug developers. Recently, drug repositioning has gained recognition as an alternative approach that explores new avenues for pre-existing commercially approved or rejected drugs to treat diseases aside from the intended ones. Drug repositioning results in lower overall developmental expenses and risk assessments, as the efficacy and safety of the original drug have already been well accessed and approved by regulatory authorities. The greatest advantage of drug repositioning is that it breathes new life into the novel, rare, orphan, and resistant diseases, such as Cushing's syndrome, HIV infection, and pandemic outbreaks such as COVID-19. Repositioning existing drugs such as Hydroxychloroquine, Remdesivir, Ivermectin and Baricitinib shows good potential for COVID-19 treatment. This can crucially aid in resolving outbreaks in urgent times of need. This review discusses the past success in drug repositioning, the current technological advancement in the field, drug repositioning for personalised medicine and the ongoing research on newly emerging drugs under consideration for the COVID-19 treatment.
Keywords: COVID-19; Cushing’s syndrome; HIV; drug personalisation; drug repositioning; novel diseases; orphan diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
[Repurposing of chlorpromazine in COVID-19 treatment: the reCoVery study].Encephale. 2020 Jun;46(3S):S35-S39. doi: 10.1016/j.encep.2020.04.010. Epub 2020 Apr 29. Encephale. 2020. PMID: 32387014 Free PMC article. French.
-
Drug repositioning is an alternative for the treatment of coronavirus COVID-19.Int J Antimicrob Agents. 2020 Jun;55(6):105969. doi: 10.1016/j.ijantimicag.2020.105969. Epub 2020 Apr 9. Int J Antimicrob Agents. 2020. PMID: 32278811 Free PMC article. Review.
-
Need for Transparency and Reliable Evidence in Emergency Use Authorizations for Coronavirus Disease 2019 (COVID-19) Therapies.JAMA Intern Med. 2020 Sep 1;180(9):1145-1146. doi: 10.1001/jamainternmed.2020.2402. JAMA Intern Med. 2020. PMID: 32427277 No abstract available.
-
Pharmacologic Therapy for COVID-19 Infection.J Community Health. 2020 Jun;45(3):435-436. doi: 10.1007/s10900-020-00821-z. J Community Health. 2020. PMID: 32314081 Free PMC article.
-
Drug Repositioning for COVID-19.Colomb Med (Cali). 2020 Jun 30;51(2):e4279. doi: 10.25100/cm.v51i2.4279. Colomb Med (Cali). 2020. PMID: 33012890 Free PMC article. Review.
Cited by
-
A Review on Measures to Rejuvenate Immune System: Natural Mode of Protection Against Coronavirus Infection.Front Immunol. 2022 Mar 15;13:837290. doi: 10.3389/fimmu.2022.837290. eCollection 2022. Front Immunol. 2022. PMID: 35371007 Free PMC article. Review.
-
Repositioning Ivermectin for Covid-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication.Biochim Biophys Acta Mol Basis Dis. 2022 Feb 1;1868(2):166294. doi: 10.1016/j.bbadis.2021.166294. Epub 2021 Oct 20. Biochim Biophys Acta Mol Basis Dis. 2022. PMID: 34687900 Free PMC article. Review.
-
Identification of Potential Tryptase Inhibitors from FDA-Approved Drugs Using Machine Learning, Molecular Docking, and Experimental Validation.ACS Omega. 2024 Sep 4;9(37):38820-38831. doi: 10.1021/acsomega.4c04886. eCollection 2024 Sep 17. ACS Omega. 2024. PMID: 39310179 Free PMC article.
-
In silico investigation of the role of vitamins in cancer therapy through inhibition of MCM7 oncoprotein.RSC Adv. 2022 Oct 31;12(48):31004-31015. doi: 10.1039/d2ra03703c. eCollection 2022 Oct 27. RSC Adv. 2022. PMID: 36349041 Free PMC article.
-
NF-κB Signaling and Inflammation-Drug Repurposing to Treat Inflammatory Disorders?Biology (Basel). 2022 Feb 26;11(3):372. doi: 10.3390/biology11030372. Biology (Basel). 2022. PMID: 35336746 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

