A Preoperative Nomogram for Predicting Chemoresistance to Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Squamous Carcinoma Treated with Radical Hysterectomy
- PMID: 32972047
- PMCID: PMC7812015
- DOI: 10.4143/crt.2020.159
A Preoperative Nomogram for Predicting Chemoresistance to Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Squamous Carcinoma Treated with Radical Hysterectomy
Abstract
Purpose: This study aimed to investigate the factors associated with chemoresistance to neoadjuvant chemotherapy (NACT) followed by radical hysterectomy (RH) and construct a nomogram to predict the chemoresistance in patients with locally advanced cervical squamous carcinoma (LACSC).
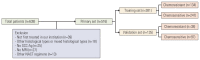
Materials and methods: This retrospective study included 516 patients with International Federation of Gynecology and Obstetrics (2003) stage IB2 and IIA2 cervical cancer treated with NACT and RH between 2007 and 2017. Clinicopathologic data were collected, and patients were assigned to training (n=381) and validation (n=135) sets. Univariate and multivariate analyses were performed to analyze factors associated with chemoresistance to NACT. A nomogram was built using the multivariate logistic regression analysis results. We evaluated the discriminative ability and accuracy of the model using a concordance index and a calibration curve. The predictive probability of chemoresistance to NACT was defined as > 34%.
Results: Multivariate analysis confirmed menopausal status, clinical tumor diameter, serum squamous cell carcinoma antigen level, and parametrial invasion on magnetic resonance imaging before treatment as independent prognostic factors associated with chemoresistance to NACT. The concordance indices of the nomogram for training and validation sets were 0.861 (95% confidence interval [CI], 0.822 to 0.900) and 0.807 (95% CI, 0.807 to 0.888), respectively. Calibration plots revealed a good fit between the modelpredicted probabilities and actual probabilities (Hosmer-Lemeshow test, p=0.597). Furthermore, grouping based on the nomogram was associated with progression-free survival.
Conclusion: We developed a nomogram for predicting chemoresistance in LACSC patients treated with RH. This nomogram can help physicians make clinical decisions regarding primary management and postoperative follow-up of the patients.
Keywords: Chemoresistance; Neoadjuvant chemotherapy; Nomograms; Uterine cervical neoplasms.
Conflict of interest statement
Conflicts of interest relevant to this article was not reported.
Figures




References
-
- Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22–36. - PubMed
-
- Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta-analysis Collaboration Neoadjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur J Cancer. 2003;39:2470–86. - PubMed
-
- Hsieh HY, Huang JW, Lu CH, Lin JC, Wang L. Definite chemoradiotherapy is a competent treatment option in FIGO stage IB2 cervical cancer compared with radical surgery +/− neoadjuvant chemotherapy. J Formos Med Assoc. 2019;118:99–108. - PubMed
-
- Katsumata N, Yoshikawa H, Kobayashi H, Saito T, Kuzuya K, Nakanishi T, et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: a Japan Clinical Oncology Group trial (JCOG 0102) Br J Cancer. 2013;108:1957–63. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

