C2-Symmetric Dinickel Catalysts for Enantioselective [4 + 1]-Cycloadditions
- PMID: 32972140
- PMCID: PMC7698671
- DOI: 10.1021/jacs.0c08262
C2-Symmetric Dinickel Catalysts for Enantioselective [4 + 1]-Cycloadditions
Abstract
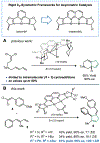
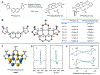
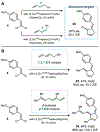
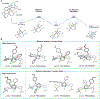
Dinickel naphthyridine-bis(oxazoline) catalysts promote enantioselective intermolecular [4 + 1]-cycloadditions of vinylidene equivalents and 1,3-dienes. The products of this reaction are methylenecyclopentenes, and the exocyclic alkene is generally obtained with high Z selectivity. E- and Z-dienes react in a stereoconvergent fashion, providing cycloadducts with the same sense of absolute stereochemistry and nearly identical ee values. This feature allows dienes that are commercially available as E/Z mixtures to be used as substrates for the cycloaddition. A DFT model for the origin of asymmetric induction is provided.
Figures

References
-
- Yoon TP; Jacobsen EN “Privileged chiral catalysts” Science 2003, 299, 1691–1693. - PubMed
-
- Ghosh AK; Mathivanan P; Cappiello J “C2-symmetric chiral bis(oxazoline)–metal complexes in catalytic asymmetric synthesis” Tetrahedron: Asymmetry 1998, 9, 1–45; - PMC - PubMed
- Johnson JS; Evans DA “Chiral bis(oxazoline) copper(II) complexes: Versatile catalysts for enantioselective cycloaddition, aldol, michael, and carbonyl ene reactions” Acc. Chem. Res 2000, 33, 325–335; - PubMed
- Fache F; Schulz E; Tommasino ML; Lemaire M “Nitrogen-containing ligands for asymmetric homogeneous and heterogeneous catalysis” Chem. Rev 2000, 100, 2159–2232; - PubMed
- Desimoni G; Faita G; Quadrelli P “Pyridine-2,6-bis(oxazolines), helpful ligands for asymmetric catalysts” Chem. Rev 2003, 103, 3119–3154; - PubMed
- Desimoni G; Faita G; Jørgensen KA “C2-symmetric chiral bis(oxazoline) ligands in asymmetric catalysis” Chem. Rev 2006, 106, 3561–3651; - PubMed
- Rasappan R; Laventine D; Reiser O “Metal-bis(oxazoline) complexes: From coordination chemistry to asymmetric catalysis” Coord. Chem. Rev 2008, 252, 702–714;
- Babu SA; Krishnan KK; Ujwaldev SM; Anilkumar G “Applications of pybox complexes in asymmetric catalysis” Asian J. Org. Chem 2018, 7, 1033–1053.
-
- Doyle MP “Perspective on dirhodium carboxamidates as catalysts” J. Org. Chem 2006, 71, 9253–9260; - PMC - PubMed
- Davies HML; Manning JR “Catalytic C-H functionalization by metal carbenoid and nitrenoid insertion” Nature 2008, 451, 417–424; - PMC - PubMed
- Davies HML; Morton D “Guiding principles for site selective and stereoselective intermolecular C-H functionalization by donor/acceptor rhodium carbenes” Chem. Soc. Rev 2011, 40, 1857–1869; - PubMed
- Paton RS; Brown JM “Dinuclear palladium complexes—precursors or catalysts?” Angew. Chem., Int. Ed 2012, 51, 10448–10450; - PubMed
- Kornecki KP; Berry JF; Powers DC; Ritter T In Prog. Inorg. Chem; Karlin KD, Ed.; John Wiley & Sons, Inc.: Hoboken, New Jersey, 2014; Vol. 58, p 225–302;
- Mankad NP “Selectivity effects in bimetallic catalysis” Chem.-Eur. J 2016, 22, 5822–5829; - PubMed
- Powers IG; Uyeda C “Metal-metal bonds in catalysis” ACS Catalysis 2017, 7, 936–958;
- Farley CM; Uyeda C “Organic reactions enabled by catalytically active metal-metal bonds” Trends in Chem. 2019, 1, 497–509.
-
- Turkenburg LAM; de Worlf WH; Bickelhaupt F “1,4-addition of dichlorocarbene to 1,2-bismethylenecycloheptane” Tetrahedron Lett. 1982, 23, 769–770;
- Sugita H; Mizuno K; Mori T; Isagawa K; Otsuji Y “Unusual mode of addition of 1,2-alkadienylidene carbenes to 1,3-dienes: 1,4 addition to rigid and flexible 1,3-dienes” Angew. Chem., Int. Ed 1991, 30, 984–986;
- Boisvert L; Beaumier F; Spino C “Evidence for a concerted [4 + 1]- cycloaddition between electron-rich carbenes and electron-deficient dienes” Org. Lett 2007, 9, 5361–5363. - PubMed
-
-
Examples of tandem cyclopropanation/1,3-rearrangement reactions:
- Danheiser RL; Martinez-Davila C; Auchus RJ; Kadonaga JT. “Stereoselective synthesis of cyclopentene derivatives from 1,3-dienes” J. Am. Chem. Soc 1981, 103, 2443–2446;
- Hudlicky T; Koszyk FF; Kutchan TM; Sheth JP “Cyclopentene annulation via intramolecular addition of diazoketones to 1,3-dienes. Applications to the synthesis of cyclopentanoid terpenes” J. Org. Chem 1980, 45, 5020–5027;
- Danheiser RL; Bronson JJ; Okano K “Carbanion-accelerated vinylcyclopropane rearrangement. Application in a general, stereocontrolled annulation approach to cyclopentene derivatives” J. Am. Chem. Soc 1985, 107, 4579–4581;
- Coscia RW; Lambert TH “Development of a formal [4 + 1] cycloaddition: Pd(OAc)2-catalyzed intramolecular cyclopropanation of 1,3-dienyl β-keto esters and MgI2-promoted vinylcyclopropane−cyclopentene rearrangement” J. Am. Chem. Soc 2009, 131, 2496–2498; - PubMed
- Beaumier F; Dupuis M; Spino C; Legault CY “Formal intramolecular (4 + 1)-cycloaddition of dialkoxycarbenes: Control of the stereoselectivity and a mechanistic portrait” J. Am. Chem. Soc 2012, 134, 5938–5953. - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

