Single-Cell RNA Sequencing Analysis Reveals a Crucial Role for CTHRC1 (Collagen Triple Helix Repeat Containing 1) Cardiac Fibroblasts After Myocardial Infarction
- PMID: 32972203
- PMCID: PMC7730974
- DOI: 10.1161/CIRCULATIONAHA.119.044557
Single-Cell RNA Sequencing Analysis Reveals a Crucial Role for CTHRC1 (Collagen Triple Helix Repeat Containing 1) Cardiac Fibroblasts After Myocardial Infarction
Abstract
Background: Cardiac fibroblasts (CFs) have a central role in the ventricular remodeling process associated with different types of fibrosis. Recent studies have shown that fibroblasts do not respond homogeneously to heart injury. Because of the limited set of bona fide fibroblast markers, a proper characterization of fibroblast population heterogeneity in response to cardiac damage is lacking. The purpose of this study was to define CF heterogeneity during ventricular remodeling and the underlying mechanisms that regulate CF function.
Methods: Collagen1α1-GFP (green fluorescent protein)-positive CFs were characterized after myocardial infarction (MI) by single-cell and bulk RNA sequencing, assay for transposase-accessible chromatin sequencing, and functional assays. Swine and patient samples were studied using bulk RNA sequencing.
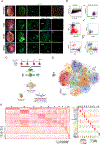
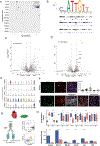
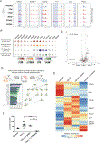
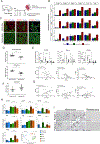
Results: We identified and characterized a unique CF subpopulation that emerges after MI in mice. These activated fibroblasts exhibit a clear profibrotic signature, express high levels of Cthrc1 (collagen triple helix repeat containing 1), and localize into the scar. Noncanonical transforming growth factor-β signaling and different transcription factors including SOX9 are important regulators mediating their response to cardiac injury. Absence of CTHRC1 results in pronounced lethality attributable to ventricular rupture. A population of CFs with a similar transcriptome was identified in a swine model of MI and in heart tissue from patients with MI and dilated cardiomyopathy.
Conclusions: We report CF heterogeneity and their dynamics during the course of MI and redefine the CFs that respond to cardiac injury and participate in myocardial remodeling. Our study identifies CTHRC1 as a novel regulator of the healing scar process and a target for future translational studies.
Keywords: fibroblasts; myocardial infarction; sequence analysis, RNA.
Conflict of interest statement
Figures


References
-
- Ruiz-Villalba A, Simon AM, Pogontke C, Castillo MI, Abizanda G, Pelacho B, Sanchez-Dominguez R, Segovia JC, Prosper F, Perez-Pomares JM. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol. 2015;65:2057–66. doi: 10.1016/j.jacc.2015.03.520 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

