Abiraterone acetate preferentially enriches for the gut commensal Akkermansia muciniphila in castrate-resistant prostate cancer patients
- PMID: 32973149
- PMCID: PMC7515896
- DOI: 10.1038/s41467-020-18649-5
Abiraterone acetate preferentially enriches for the gut commensal Akkermansia muciniphila in castrate-resistant prostate cancer patients
Erratum in
-
Author Correction: Abiraterone acetate preferentially enriches for the gut commensal Akkermansia muciniphila in castrate-resistant prostate cancer patients.Nat Commun. 2020 Dec 9;11(1):6394. doi: 10.1038/s41467-020-20410-x. Nat Commun. 2020. PMID: 33298912 Free PMC article. No abstract available.
Abstract
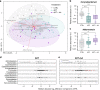
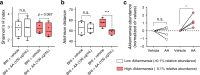
Abiraterone acetate (AA) is an inhibitor of androgen biosynthesis, though this cannot fully explain its efficacy against androgen-independent prostate cancer. Here, we demonstrate that androgen deprivation therapy depletes androgen-utilizing Corynebacterium spp. in prostate cancer patients and that oral AA further enriches for the health-associated commensal, Akkermansia muciniphila. Functional inferencing elucidates a coinciding increase in bacterial biosynthesis of vitamin K2 (an inhibitor of androgen dependent and independent tumor growth). These results are highly reproducible in a host-free gut model, excluding the possibility of immune involvement. Further investigation reveals that AA is metabolized by bacteria in vitro and that breakdown components selectively impact growth. We conclude that A. muciniphila is a key regulator of AA-mediated restructuring of microbial communities, and that this species may affect treatment response in castrate-resistant cohorts. Ongoing initiatives aimed at modulating the colonic microbiota of cancer patients may consider targeted delivery of poorly absorbed selective bacterial growth agents.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

