Aldosterone up-regulates voltage-gated potassium currents and NKCC1 protein membrane fractions
- PMID: 32973172
- PMCID: PMC7515911
- DOI: 10.1038/s41598-020-72450-4
Aldosterone up-regulates voltage-gated potassium currents and NKCC1 protein membrane fractions
Abstract
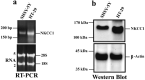
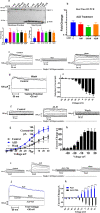
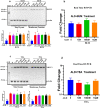
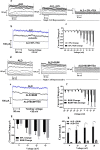
Na+-K+-2Cl- Cotransporter (NKCC1) is a protein that aids in the active transport of sodium, potassium, and chloride ions across cell membranes. It has been shown that long-term systemic treatment with aldosterone (ALD) can enhance NKCC1 protein expression and activity in the aging cochlea resulting in improved hearing. In the present work, we used a cell line with confirmed NKCC1 expression to demonstrate that in vitro application of ALD increased outward voltage-gated potassium currents significantly, and simultaneously upregulated whole lysate and membrane portion NKCC1 protein expression. These ALD-induced changes were blocked by applying the mineralocorticoid receptor antagonist eplerenone. However, application of the NKCC1 inhibitor bumetanide or the potassium channel antagonist Tetraethyl ammonium had no effect. In addition, NKKC1 mRNA levels remained stable, indicating that ALD modulates NKCC1 protein expression via the activation of mineralocorticoid receptors and post-transcriptional modifications. Further, in vitro electrophysiology experiments, with ALD in the presence of NKCC1, K+ channel and mineralocorticoid receptor inhibitors, revealed interactions between NKCC1 and outward K+ channels, mediated by a mineralocorticoid receptor-ALD complex. These results provide evidence of the therapeutic potential of ALD for the prevention/treatment of inner ear disorders such as age-related hearing loss.
Conflict of interest statement
Drs. Ding, Frisina, Zhu and Walton hold a patent (not yet licensed) on the use of aldosterone and anti-inflammatories to treat age-related hearing loss. There are no other actual or perceived conflicts for the authors of this manuscript regarding funding source agencies, NIH or others, for the research reported in this manuscript.
Figures

Similar articles
-
Aldosterone up-regulates basolateral Na+ -K+ -2Cl- cotransporter-1 to support enhanced large-conductance K+ channel-mediated K+ secretion in rat distal colon.FASEB J. 2021 May;35(5):e21606. doi: 10.1096/fj.202100203R. FASEB J. 2021. PMID: 33908679 Free PMC article.
-
Direct control of Na(+)-K(+)-2Cl(-)-cotransport protein (NKCC1) expression with aldosterone.Am J Physiol Cell Physiol. 2014 Jan 1;306(1):C66-75. doi: 10.1152/ajpcell.00096.2013. Epub 2013 Oct 30. Am J Physiol Cell Physiol. 2014. PMID: 24173102 Free PMC article.
-
Aldosterone regulates the Na-K-2Cl cotransporter in vascular smooth muscle.Hypertension. 2003 May;41(5):1131-5. doi: 10.1161/01.HYP.0000066128.04083.CA. Epub 2003 Mar 31. Hypertension. 2003. PMID: 12668585
-
The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures.Neurosurg Focus. 2008 Sep;25(3):E22. doi: 10.3171/FOC/2008/25/9/E22. Neurosurg Focus. 2008. PMID: 18759624 Review.
-
The role of Na-K-Cl co-transporter in cerebral ischemia.Neurol Res. 2005 Apr;27(3):280-6. doi: 10.1179/016164105X25243. Neurol Res. 2005. PMID: 15845211 Review.
Cited by
-
Dietary Na+ depletion up-regulates NKCC1 expression and enhances electrogenic Cl- secretion in rat proximal colon.Cell Mol Life Sci. 2023 Jul 17;80(8):209. doi: 10.1007/s00018-023-04857-x. Cell Mol Life Sci. 2023. PMID: 37458846 Free PMC article.
-
Aldosterone up-regulates basolateral Na+ -K+ -2Cl- cotransporter-1 to support enhanced large-conductance K+ channel-mediated K+ secretion in rat distal colon.FASEB J. 2021 May;35(5):e21606. doi: 10.1096/fj.202100203R. FASEB J. 2021. PMID: 33908679 Free PMC article.
-
Loss of central mineralocorticoid or glucocorticoid receptors impacts auditory nerve processing in the cochlea.iScience. 2022 Feb 26;25(3):103981. doi: 10.1016/j.isci.2022.103981. eCollection 2022 Mar 18. iScience. 2022. PMID: 35281733 Free PMC article.
-
The Roles of Solute Carriers in Auditory Function.Front Genet. 2022 Jan 26;13:823049. doi: 10.3389/fgene.2022.823049. eCollection 2022. Front Genet. 2022. PMID: 35154281 Free PMC article. Review.
-
Connexins 30 and 43 expression changes in relation to age-related hearing loss.Hear Res. 2024 Mar 15;444:108971. doi: 10.1016/j.heares.2024.108971. Epub 2024 Feb 11. Hear Res. 2024. PMID: 38359484 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

