The neuropathic phenotype of the K/BxN transgenic mouse with spontaneous arthritis: pain, nerve sprouting and joint remodeling
- PMID: 32973194
- PMCID: PMC7515905
- DOI: 10.1038/s41598-020-72441-5
The neuropathic phenotype of the K/BxN transgenic mouse with spontaneous arthritis: pain, nerve sprouting and joint remodeling
Abstract
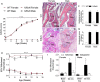
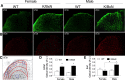
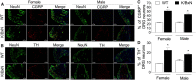
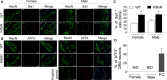
The adult K/BxN transgenic mouse develops spontaneous autoimmune arthritis with joint remodeling and profound bone loss. We report that both males and females display a severe sustained tactile allodynia which is reduced by gabapentin but not the potent cyclooxygenase inhibitor ketorolac. In dorsal horn, males and females show increased GFAP+ astrocytic cells; however, only males demonstrate an increase in Iba1+ microglia. In dorsal root ganglia (DRG), there is an increase in CGRP+, TH+, and Iba1+ (macrophage) labeling, but no increase in ATF3+ cells. At the ankle there is increased CGRP+, TH+, and GAP-43+ fiber synovial innervation. Thus, based on the changes in dorsal horn, DRG and peripheral innervation, we suggest that the adult K/BxN transgenic arthritic mice display a neuropathic phenotype, an assertion consistent with the analgesic pharmacology seen in this animal. These results indicate the relevance of this model to our understanding of the nociceptive processing which underlies the chronic pain state that evolves secondary to persistent joint inflammation.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Role of TLR4 activation and signaling in bone remodeling, and afferent sprouting in serum transfer arthritis.Arthritis Res Ther. 2024 Dec 18;26(1):212. doi: 10.1186/s13075-024-03424-4. Arthritis Res Ther. 2024. PMID: 39696684 Free PMC article.
-
Characterization of the acute and persistent pain state present in K/BxN serum transfer arthritis.Pain. 2010 Nov;151(2):394-403. doi: 10.1016/j.pain.2010.07.030. Epub 2010 Aug 23. Pain. 2010. PMID: 20739123 Free PMC article.
-
Evaluation of the neonatal streptozotocin model of diabetes in rats: Evidence for a model of neuropathic pain.Pharmacol Rep. 2018 Apr;70(2):294-303. doi: 10.1016/j.pharep.2017.09.002. Epub 2017 Sep 14. Pharmacol Rep. 2018. PMID: 29477037 Free PMC article.
-
Sigma-1 receptors control neuropathic pain and macrophage infiltration into the dorsal root ganglion after peripheral nerve injury.FASEB J. 2020 Apr;34(4):5951-5966. doi: 10.1096/fj.201901921R. Epub 2020 Mar 10. FASEB J. 2020. PMID: 32157739
-
The Different Dynamic Changes of Nerve Growth Factor in the Dorsal Horn and Dorsal Root Ganglion Leads to Hyperalgesia and Allodynia in Diabetic Neuropathic Pain.Pain Physician. 2017 May;20(4):E551-E561. Pain Physician. 2017. PMID: 28535564
Cited by
-
Role of TLR4 activation and signaling in bone remodeling, and afferent sprouting in serum transfer arthritis.Arthritis Res Ther. 2024 Dec 18;26(1):212. doi: 10.1186/s13075-024-03424-4. Arthritis Res Ther. 2024. PMID: 39696684 Free PMC article.
-
Synovial fibroblast gene expression is associated with sensory nerve growth and pain in rheumatoid arthritis.Sci Transl Med. 2024 Apr 10;16(742):eadk3506. doi: 10.1126/scitranslmed.adk3506. Epub 2024 Apr 10. Sci Transl Med. 2024. PMID: 38598614 Free PMC article.
-
Altered sensory innervation and pain hypersensitivity in a model of young painful arthritic joints: short- and long-term effects.Inflamm Res. 2021 Apr;70(4):483-493. doi: 10.1007/s00011-021-01450-5. Epub 2021 Mar 13. Inflamm Res. 2021. PMID: 33715021 Free PMC article.
-
Eosinophils preserve bone homeostasis by inhibiting excessive osteoclast formation and activity via eosinophil peroxidase.Nat Commun. 2024 Feb 5;15(1):1067. doi: 10.1038/s41467-024-45261-8. Nat Commun. 2024. PMID: 38316791 Free PMC article.
-
The gut-joint axis in rheumatoid arthritis.Nat Rev Rheumatol. 2021 Apr;17(4):224-237. doi: 10.1038/s41584-021-00585-3. Epub 2021 Mar 5. Nat Rev Rheumatol. 2021. PMID: 33674813 Review.
References
-
- Wolfe F, Michaud K, Li T. Sleep disturbance in patients with rheumatoid arthritis: Evaluation by medical outcomes study and visual analog sleep scales. J. Rheumatol. 2006;33:1942–1951. - PubMed
-
- Sambrook PN. The skeleton in rheumatoid arthritis: Common mechanisms for bone erosion and osteoporosis? J. Rheumatol. 2000;27:2541–2542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

