Cyclic AMP efflux through MRP4 regulates actin dynamics signalling pathway and sperm motility in bovines
- PMID: 32973195
- PMCID: PMC7518284
- DOI: 10.1038/s41598-020-72425-5
Cyclic AMP efflux through MRP4 regulates actin dynamics signalling pathway and sperm motility in bovines
Abstract
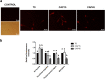
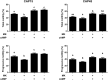
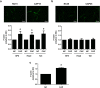
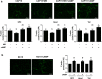
Previously we demonstrated that multidrug resistance-associated protein 4 transporter (MRP4) mediates cAMP efflux in bovine spermatozoa and that extracellular cAMP (ecAMP) triggers events associated to capacitation. Here, we deepen the study of the role of MRP4 in bovine sperm function by using MK571, an MRP4 inhibitor. The incubation of spermatozoa with MK571 during 45 min inhibited capacitation-associated events. MRP4 was localized in post-acrosomal region and mid-piece at 15 min capacitation, while at 45 min it was mainly located in the acrosome. After 15 min, MK571 decreased total sperm motility (TM), progressive motility (PM) and several kinematic parameters. The addition of ecAMP rescued MK571 effect and ecAMP alone increased the percentage of motile sperm and kinematics parameters. Since actin cytoskeleton plays essential roles in the regulation of sperm motility, we investigated if MRP4 activity might affect actin polymerization. After 15 min capacitation, an increase in F-actin was observed, which was inhibited by MK571. This effect was reverted by the addition of ecAMP. Furthermore, ecAMP alone increased F-actin levels while no F-actin was detected with ecAMP in the presence of PKA inhibitors. Our results support the importance of cAMP efflux through MRP4 in sperm capacitation and suggest its involvement in the regulation of actin polymerization and motility.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
MRP4-mediated cAMP efflux is essential for mouse spermatozoa capacitation.J Cell Sci. 2019 Jul 26;132(14):jcs230565. doi: 10.1242/jcs.230565. J Cell Sci. 2019. PMID: 31253671
-
Cyclic AMP efflux, via MRPs and A1 adenosine receptors, is critical for bovine sperm capacitation.Mol Hum Reprod. 2014 Jan;20(1):89-99. doi: 10.1093/molehr/gat053. Epub 2013 Aug 1. Mol Hum Reprod. 2014. PMID: 23907162
-
Extracellular cAMP activates molecular signalling pathways associated with sperm capacitation in bovines.Mol Hum Reprod. 2017 Aug 1;23(8):521-534. doi: 10.1093/molehr/gax030. Mol Hum Reprod. 2017. PMID: 28521061
-
Regulation of Sperm Capacitation and the Acrosome Reaction by PIP 2 and Actin Modulation.Asian J Androl. 2015 Jul-Aug;17(4):597-600. doi: 10.4103/1008-682X.154305. Asian J Androl. 2015. PMID: 25966627 Free PMC article. Review.
-
Signaling pathways in sperm capacitation and acrosome reaction.Cell Mol Biol (Noisy-le-grand). 2003 May;49(3):321-7. Cell Mol Biol (Noisy-le-grand). 2003. PMID: 12887084 Review.
Cited by
-
Modulation of Cyclic AMP Levels in Fallopian Tube Cells by Natural and Environmental Estrogens.Cells. 2021 May 19;10(5):1250. doi: 10.3390/cells10051250. Cells. 2021. PMID: 34069403 Free PMC article.
-
The Modulation of Functional Status of Bovine Spermatozoa by Progesterone.Animals (Basel). 2021 Jun 15;11(6):1788. doi: 10.3390/ani11061788. Animals (Basel). 2021. PMID: 34203892 Free PMC article.
-
Comparative transcriptome analysis of bull X- and Y-spermatozoa.Sci Rep. 2025 Apr 26;15(1):14593. doi: 10.1038/s41598-025-99438-2. Sci Rep. 2025. PMID: 40287506 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

