Distinguish fatty acids impact survival, differentiation and cellular function of periodontal ligament fibroblasts
- PMID: 32973207
- PMCID: PMC7518255
- DOI: 10.1038/s41598-020-72736-7
Distinguish fatty acids impact survival, differentiation and cellular function of periodontal ligament fibroblasts
Abstract
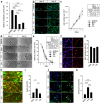
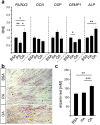
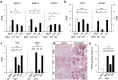
Alveolar bone (AB) remodeling is necessary for the adaption to mechanical stimuli occurring during mastication and orthodontic tooth movement (OTM). Thereby, bone degradation and assembly are strongly regulated processes that can be altered in obese patients. Further, increased fatty acids (FA) serum levels affect bone remodeling cells and we, therefore, investigated whether they also influence the function of periodontal ligament fibroblast (PdLF). PdLF are a major cell type regulating the differentiation and function of osteoblasts and osteoclasts localized in the AB. We stimulated human PdLF (HPdLF) in vitro with palmitic (PA) or oleic acid (OA) and analyzed their metabolic activity, growth, survival and expression of osteogenic markers and calcium deposits. Our results emphasize that PA increased cell death of HPdLF, whereas OA induced their osteoblastic differentiation. Moreover, quantitative expression analysis of OPG and RANKL revealed altered levels in mechanically stimulated PA-treated HPdLF. Furthermore, osteoclasts stimulated with culture medium of mechanical stressed FA-treated HPdLF revealed significant changes in cell differentiation upon FA-treatment. For the first time, our results highlight a potential role of specific FA in the function of HPdLF-modulated AB remodeling and help to elucidate the complex interplay of bone metabolism, mechanical stimulation and obesity-induced alterations.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Hyperlipidemic Conditions Impact Force-Induced Inflammatory Response of Human Periodontal Ligament Fibroblasts Concomitantly Challenged with P. gingivalis-LPS.Int J Mol Sci. 2021 Jun 4;22(11):6069. doi: 10.3390/ijms22116069. Int J Mol Sci. 2021. PMID: 34199865 Free PMC article.
-
Osteogenic differentiation of periodontal fibroblasts is dependent on the strength of mechanical strain.Arch Oral Biol. 2013 Jul;58(7):896-904. doi: 10.1016/j.archoralbio.2013.01.009. Epub 2013 Feb 17. Arch Oral Biol. 2013. PMID: 23422327
-
Diet-Induced Obesity and Its Differential Impact on Periodontal Bone Loss.J Dent Res. 2016 Feb;95(2):223-9. doi: 10.1177/0022034515609882. Epub 2015 Oct 8. J Dent Res. 2016. PMID: 26450512 Free PMC article.
-
Role of periodontal ligament fibroblasts in osteoclastogenesis: a review.J Periodontal Res. 2015 Apr;50(2):152-9. doi: 10.1111/jre.12197. Epub 2014 May 24. J Periodontal Res. 2015. PMID: 24862732 Review.
-
Estrogen effects on orthodontic tooth movement and orthodontically-induced root resorption.Arch Oral Biol. 2020 Oct;118:104840. doi: 10.1016/j.archoralbio.2020.104840. Epub 2020 Jul 18. Arch Oral Biol. 2020. PMID: 32730908 Review.
Cited by
-
Bidirectional substance P signaling between periodontal ligament fibroblasts and sensory neurons under mechanical stress.Front Mol Neurosci. 2025 May 16;18:1583908. doi: 10.3389/fnmol.2025.1583908. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40453756 Free PMC article.
-
Gut microbiome dysbiosis in patients with hepatitis B virus-related hepatocellular carcinoma after extended hepatectomy liver failure.Ann Transl Med. 2022 May;10(10):549. doi: 10.21037/atm-22-1958. Ann Transl Med. 2022. PMID: 35722392 Free PMC article.
-
Hyperlipidemic Conditions Impact Force-Induced Inflammatory Response of Human Periodontal Ligament Fibroblasts Concomitantly Challenged with P. gingivalis-LPS.Int J Mol Sci. 2021 Jun 4;22(11):6069. doi: 10.3390/ijms22116069. Int J Mol Sci. 2021. PMID: 34199865 Free PMC article.
-
BML-111 inhibit H2O2-induced pyroptosis and osteogenic dysfunction of human periodontal ligament fibroblasts by activating the Nrf2/HO-1 pathway.BMC Oral Health. 2024 Jan 8;24(1):40. doi: 10.1186/s12903-023-03827-w. BMC Oral Health. 2024. PMID: 38191432 Free PMC article.
-
Palmitate-Triggered COX2/PGE2-Related Hyperinflammation in Dual-Stressed PdL Fibroblasts Is Mediated by Repressive H3K27 Trimethylation.Cells. 2022 Mar 10;11(6):955. doi: 10.3390/cells11060955. Cells. 2022. PMID: 35326406 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

