Stat2 stability regulation: an intersection between immunity and carcinogenesis
- PMID: 32973222
- PMCID: PMC8080578
- DOI: 10.1038/s12276-020-00506-6
Stat2 stability regulation: an intersection between immunity and carcinogenesis
Abstract
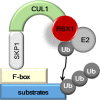
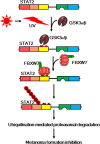
Signal transducer and activator of transcription (STAT2) is a member of the STAT family that plays an essential role in immune responses to extracellular and intracellular stimuli, including inflammatory reactions, invasion of foreign materials, and cancer initiation. Although the majority of STAT2 studies in the last few decades have focused on interferon (IFN)-α/β (IFNα/β) signaling pathway-mediated host defense against viral infections, recent studies have revealed that STAT2 also plays an important role in human cancer development. Notably, strategic research on STAT2 function has provided evidence that transient regulatory activity by homo- or heterodimerization induces its nuclear localization where it to forms a ternary IFN-stimulated gene factor 3 (ISGF3) complex, which is composed of STAT1 and/or STAT2 and IFN regulatory factor 9 (IEF9). The molecular mechanisms of ISGF3-mediated ISG gene expression provide the basic foundation for the regulation of STAT2 protein activity but not protein quality control. Recently, previously unknown molecular mechanisms of STAT2-mediated cell proliferation via STAT2 protein quality control were elucidated. In this review, we briefly summarize the role of STAT2 in immune responses and carcinogenesis with respect to the molecular mechanisms of STAT2 stability regulation via the proteasomal degradation pathway.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Type I interferon-regulated gene expression and signaling in murine mixed glial cells lacking signal transducers and activators of transcription 1 or 2 or interferon regulatory factor 9.J Biol Chem. 2017 Apr 7;292(14):5845-5859. doi: 10.1074/jbc.M116.756510. Epub 2017 Feb 17. J Biol Chem. 2017. PMID: 28213522 Free PMC article.
-
Unphosphorylated ISGF3 drives constitutive expression of interferon-stimulated genes to protect against viral infections.Sci Signal. 2017 Apr 25;10(476):eaah4248. doi: 10.1126/scisignal.aah4248. Sci Signal. 2017. PMID: 28442624
-
STAT2/IRF9 directs a prolonged ISGF3-like transcriptional response and antiviral activity in the absence of STAT1.Biochem J. 2015 Mar 15;466(3):511-24. doi: 10.1042/BJ20140644. Biochem J. 2015. PMID: 25564224 Free PMC article.
-
A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses.Front Immunol. 2018 May 28;9:1135. doi: 10.3389/fimmu.2018.01135. eCollection 2018. Front Immunol. 2018. PMID: 29892288 Free PMC article. Review.
-
The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses.Cytokine Growth Factor Rev. 2016 Jun;29:71-81. doi: 10.1016/j.cytogfr.2016.02.010. Epub 2016 Mar 18. Cytokine Growth Factor Rev. 2016. PMID: 27053489 Review.
Cited by
-
Characterization of Mucosal Immune-Related lncRNAs and mRNAs in a Mouse Model of Allergic Conjunctivitis.J Inflamm Res. 2025 May 8;18:6061-6076. doi: 10.2147/JIR.S511048. eCollection 2025. J Inflamm Res. 2025. PMID: 40357374 Free PMC article.
-
Molecular insights into type I interferon suppression and enhanced pathogenicity by species B human adenoviruses B7 and B14.mBio. 2024 Aug 14;15(8):e0103824. doi: 10.1128/mbio.01038-24. Epub 2024 Jun 28. mBio. 2024. PMID: 38940561 Free PMC article.
-
Bioinformatics Analysis of Expression Profiles and Prognostic Values of the Signal Transducer and Activator of Transcription Family Genes in Glioma.Front Genet. 2021 Jul 2;12:625234. doi: 10.3389/fgene.2021.625234. eCollection 2021. Front Genet. 2021. PMID: 34276757 Free PMC article.
-
Identifying differentially expressed genes in goat mammary epithelial cells induced by overexpression of SOCS3 gene using RNA sequencing.Front Vet Sci. 2024 May 21;11:1392152. doi: 10.3389/fvets.2024.1392152. eCollection 2024. Front Vet Sci. 2024. PMID: 38835896 Free PMC article.
-
Initial activation of STAT2 induced by IAV infection is critical for innate antiviral immunity.Front Immunol. 2022 Sep 5;13:960544. doi: 10.3389/fimmu.2022.960544. eCollection 2022. Front Immunol. 2022. PMID: 36148221 Free PMC article.
References
-
- Tanaka T, Soriano MA, Grusby MJ. SLIM is a nuclear ubiquitin E3 ligase that negatively regulates STAT signaling. Immunity. 2005;22:729–736. - PubMed
-
- Ulane CM, Horvath CM. Paramyxoviruses SV5 and HPIV2 assemble STAT protein ubiquitin ligase complexes from cellular components. Virology. 2002;304:160–166. - PubMed
-
- Garcin D, Marq JB, Strahle L, le Mercier P, Kolakofsky D. All four Sendai Virus C proteins bind Stat1, but only the larger forms also induce its mono-ubiquitination and degradation. Virology. 2002;295:256–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

