Inspired by the human placenta: a novel 3D bioprinted membrane system to create barrier models
- PMID: 32973223
- PMCID: PMC7515925
- DOI: 10.1038/s41598-020-72559-6
Inspired by the human placenta: a novel 3D bioprinted membrane system to create barrier models
Abstract
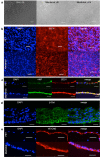
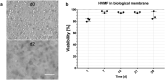
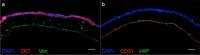
Barrier organ models need a scaffold structure to create a two compartment culture. Technical filter membranes used most often as scaffolds may impact cell behaviour and present a barrier themselves, ultimately limiting transferability of test results. In this work we present an alternative for technical filter membrane systems: a 3D bioprinted biological membrane in 24 well format. The biological membrane, based on extracellular matrix (ECM), is highly permeable and presents a natural 3D environment for cell culture. Inspired by the human placenta we established a coculture of a trophoblast-derived cell line (BeWo b30), together with primary placental fibroblasts within the biological membrane (simulating villous stroma) and primary human placental endothelial cells-representing three cellular components of the human placental villus. All cell types maintained their cell type specific marker expression after two weeks of coculture on the biological membrane. In permeability assays the trophoblast layer developed a barrier on the biological membrane, which was even more pronounced when cocultured with fibroblasts. In this work we present a filter membrane free scaffold, we characterize its properties and assess its suitability for cell culture and barrier models. Further we show a novel placenta inspired model in a complex bioprinted coculture. In the absence of an artificial filter membrane, we demonstrate barrier architecture and functionality.
Conflict of interest statement
AEK, AB, CP, AT, MAG, TL and AKA are employees at Cellbricks GmbH. LK is shareholder of Cellbricks GmbH. Rest of authors declare no competing interests.
Figures



Similar articles
-
Placenta-on-a-chip: a novel platform to study the biology of the human placenta.J Matern Fetal Neonatal Med. 2016;29(7):1046-54. doi: 10.3109/14767058.2015.1038518. Epub 2015 Jun 15. J Matern Fetal Neonatal Med. 2016. PMID: 26075842 Free PMC article.
-
Distribution of human endogenous retrovirus type W receptor in normal human villous placenta.Pathology. 2007 Aug;39(4):406-12. doi: 10.1080/00313020701444572. Pathology. 2007. PMID: 17676482
-
Establishment of an in vitro placental barrier model cultured under physiologically relevant oxygen levels.Mol Hum Reprod. 2020 May 15;26(5):353-365. doi: 10.1093/molehr/gaaa018. Mol Hum Reprod. 2020. PMID: 32159799 Free PMC article.
-
Cellular Models of Trophoblast Differentiation.Semin Reprod Med. 2016 Jan;34(1):50-6. doi: 10.1055/s-0035-1570026. Epub 2016 Jan 11. Semin Reprod Med. 2016. PMID: 26752716 Review.
-
Macrophage migration inhibitory factor in human early pregnancy events and association with placental pathologies.Placenta. 2021 Dec;116:51-57. doi: 10.1016/j.placenta.2021.02.007. Epub 2021 Feb 15. Placenta. 2021. PMID: 33612316 Review.
Cited by
-
Generation of a pancreas derived hydrogel for the culture of hiPSC derived pancreatic endocrine cells.Sci Rep. 2024 Sep 4;14(1):20653. doi: 10.1038/s41598-024-67327-9. Sci Rep. 2024. PMID: 39232042 Free PMC article.
-
Overview of Drug Transporters in Human Placenta.Int J Mol Sci. 2021 Dec 5;22(23):13149. doi: 10.3390/ijms222313149. Int J Mol Sci. 2021. PMID: 34884954 Free PMC article. Review.
-
Are the Organoid Models an Invaluable Contribution to ZIKA Virus Research?Pathogens. 2021 Sep 24;10(10):1233. doi: 10.3390/pathogens10101233. Pathogens. 2021. PMID: 34684182 Free PMC article. Review.
-
Modelling the Human Placental Interface In Vitro-A Review.Micromachines (Basel). 2021 Jul 27;12(8):884. doi: 10.3390/mi12080884. Micromachines (Basel). 2021. PMID: 34442506 Free PMC article. Review.
-
Beyond 2D: Novel biomaterial approaches for modeling the placenta.Placenta. 2024 Nov;157:55-66. doi: 10.1016/j.placenta.2024.03.006. Epub 2024 Mar 14. Placenta. 2024. PMID: 38514278 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

