Spatial mapping of the collagen distribution in human and mouse tissues by force volume atomic force microscopy
- PMID: 32973235
- PMCID: PMC7518416
- DOI: 10.1038/s41598-020-72564-9
Spatial mapping of the collagen distribution in human and mouse tissues by force volume atomic force microscopy
Abstract
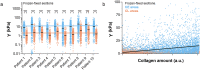
Changes in the elastic properties of living tissues during normal development and in pathological processes are often due to modifications of the collagen component of the extracellular matrix at various length scales. Force volume AFM can precisely capture the mechanical properties of biological samples with force sensitivity and spatial resolution. The integration of AFM data with data of the molecular composition contributes to understanding the interplay between tissue biochemistry, organization and function. The detection of micrometer-size, heterogeneous domains at different elastic moduli in tissue sections by AFM has remained elusive so far, due to the lack of correlations with histological, optical and biochemical assessments. In this work, force volume AFM is used to identify collagen-enriched domains, naturally present in human and mouse tissues, by their elastic modulus. Collagen identification is obtained in a robust way and affordable timescales, through an optimal design of the sample preparation method and AFM parameters for faster scan with micrometer resolution. The choice of a separate reference sample stained for collagen allows correlating elastic modulus with collagen amount and position with high statistical significance. The proposed preparation method ensures safe handling of the tissue sections guarantees the preservation of their micromechanical characteristics over time and makes it much easier to perform correlation experiments with different biomarkers independently.
Conflict of interest statement
The authors declare no competing interests.
Figures