The Use of Induced Pluripotent Stem Cells as a Model for Developmental Eye Disorders
- PMID: 32973457
- PMCID: PMC7468397
- DOI: 10.3389/fncel.2020.00265
The Use of Induced Pluripotent Stem Cells as a Model for Developmental Eye Disorders
Abstract
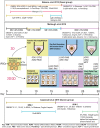
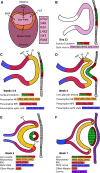
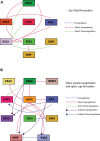
Approximately one-third of childhood blindness is attributed to developmental eye disorders, of which 80% have a genetic cause. Eye morphogenesis is tightly regulated by a highly conserved network of transcription factors when disrupted by genetic mutations can result in severe ocular malformation. Human-induced pluripotent stem cells (hiPSCs) are an attractive tool to study early eye development as they are more physiologically relevant than animal models, can be patient-specific and their use does not elicit the ethical concerns associated with human embryonic stem cells. The generation of self-organizing hiPSC-derived optic cups is a major advancement to understanding mechanisms of ocular development and disease. Their development in vitro has been found to mirror that of the human eye and these early organoids have been used to effectively model microphthalmia caused by a VSX2 variant. hiPSC-derived optic cups, retina, and cornea organoids are powerful tools for future modeling of disease phenotypes and will enable a greater understanding of the pathophysiology of many other developmental eye disorders. These models will also provide an effective platform for identifying molecular therapeutic targets and for future clinical applications.
Keywords: VSX2; corneal hereditary endothelial dystrophy; developmental eye disorders; disease modeling; eye development; human induced pluripotent stem cells; microphthalmia; ocular maldevelopment.
Copyright © 2020 Eintracht, Toms and Moosajee.
Figures


Similar articles
-
Efficient embryoid-based method to improve generation of optic vesicles from human induced pluripotent stem cells.F1000Res. 2022 Mar 17;11:324. doi: 10.12688/f1000research.108829.1. eCollection 2022. F1000Res. 2022. PMID: 35811797 Free PMC article.
-
Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2.Stem Cells. 2014 Jun;32(6):1480-92. doi: 10.1002/stem.1667. Stem Cells. 2014. PMID: 24532057 Free PMC article.
-
Animal and cellular models of microphthalmia.Ther Adv Rare Dis. 2021 Feb 27;2:2633004021997447. doi: 10.1177/2633004021997447. eCollection 2021 Jan-Dec. Ther Adv Rare Dis. 2021. PMID: 37181112 Free PMC article. Review.
-
The Role of FGF9 in the Production of Neural Retina and RPE in a Pluripotent Stem Cell Model of Early Human Retinal Development.Am J Ophthalmol. 2019 Oct;206:113-131. doi: 10.1016/j.ajo.2019.04.033. Epub 2019 May 10. Am J Ophthalmol. 2019. PMID: 31078532 Free PMC article.
-
Pluripotent Stem Cells to Model Degenerative Retinal Diseases: The RPE Perspective.Adv Exp Med Biol. 2019;1186:1-31. doi: 10.1007/978-3-030-28471-8_1. Adv Exp Med Biol. 2019. PMID: 31654384 Review.
Cited by
-
Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology.Cells. 2022 Nov 6;11(21):3516. doi: 10.3390/cells11213516. Cells. 2022. PMID: 36359912 Free PMC article. Review.
-
Establishing Functional Retina in a Dish: Progress and Promises of Induced Pluripotent Stem Cell-Based Retinal Neuron Differentiation.Int J Mol Sci. 2023 Sep 4;24(17):13652. doi: 10.3390/ijms241713652. Int J Mol Sci. 2023. PMID: 37686457 Free PMC article. Review.
-
Minigene Splicing Assays and Long-Read Sequencing to Unravel Pathogenic Deep-Intronic Variants in PAX6 in Congenital Aniridia.Int J Mol Sci. 2023 Jan 13;24(2):1562. doi: 10.3390/ijms24021562. Int J Mol Sci. 2023. PMID: 36675087 Free PMC article.
-
Genetic and environmental factors contributing to anophthalmia and microphthalmia: Current understanding and future directions.World J Clin Pediatr. 2025 Jun 9;14(2):101982. doi: 10.5409/wjcp.v14.i2.101982. eCollection 2025 Jun 9. World J Clin Pediatr. 2025. PMID: 40491727 Free PMC article. Review.
-
Inducible Pluripotent Stem Cells to Model and Treat Inherited Degenerative Diseases of the Outer Retina: 3D-Organoids Limitations and Bioengineering Solutions.Cells. 2021 Sep 20;10(9):2489. doi: 10.3390/cells10092489. Cells. 2021. PMID: 34572137 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

