NLRP3 Is Involved in the Maintenance of Cerebral Pericytes
- PMID: 32973459
- PMCID: PMC7473034
- DOI: 10.3389/fncel.2020.00276
NLRP3 Is Involved in the Maintenance of Cerebral Pericytes
Abstract
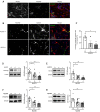
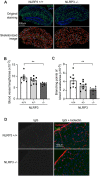
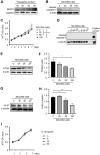
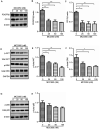
Pericytes play a central role in regulating the structure and function of capillaries in the brain. However, molecular mechanisms that drive pericyte proliferation and differentiation are unclear. In our study, we immunostained NACHT, LRR and PYD domains-containing protein 3 (NLRP3)-deficient and wild-type littermate mice and observed that NLRP3 deficiency reduced platelet-derived growth factor receptor β (PDGFRβ)-positive pericytes and collagen type IV immunoreactive vasculature in the brain. In Western blot analysis, PDGFRβ and CD13 proteins in isolated cerebral microvessels from the NLRP3-deficient mouse brain were decreased. We further treated cultured pericytes with NLRP3 inhibitor, MCC950, and demonstrated that NLRP3 inhibition attenuated cell proliferation but did not induce apoptosis. NLRP3 inhibition also decreased protein levels of PDGFRβ and CD13 in cultured pericytes. On the contrary, treatments with IL-1β, the major product of NLRP3-contained inflammasome, increased protein levels of PDGFRβ, and CD13 in cultured cells. The alteration of PDGFRβ and CD13 protein levels were correlated with the phosphorylation of AKT. Inhibition of AKT reduced both protein markers and abolished the effect of IL-1β activation in cultured pericytes. Thus, NLRP3 activation might be essential to maintain pericytes in the healthy brain through phosphorylating AKT. The potential adverse effects on the cerebral vascular pericytes should be considered in clinical therapies with NLRP3 inhibitors.
Keywords: Alzheimer’s disease; NLRP3; cerebral perfusion; neuroinflammation; pericyte.
Copyright © 2020 Quan, Luo, Tang, Furihata, Li, Fassbender and Liu.
Figures

Similar articles
-
Deficiency of NLRP3 protects cerebral pericytes and attenuates Alzheimer's pathology in tau-transgenic mice.Front Cell Neurosci. 2024 Oct 30;18:1471005. doi: 10.3389/fncel.2024.1471005. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39539344 Free PMC article.
-
Possible involvement of basic FGF in the upregulation of PDGFRβ in pericytes after ischemic stroke.Brain Res. 2016 Jan 1;1630:98-108. doi: 10.1016/j.brainres.2015.11.003. Epub 2015 Nov 10. Brain Res. 2016. PMID: 26569132
-
Involvement of platelet-derived growth factor receptor β in fibrosis through extracellular matrix protein production after ischemic stroke.Exp Neurol. 2015 Feb;264:127-34. doi: 10.1016/j.expneurol.2014.12.007. Epub 2014 Dec 13. Exp Neurol. 2015. PMID: 25510317
-
Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling.Mol Neurodegener. 2010 Aug 25;5:32. doi: 10.1186/1750-1326-5-32. Mol Neurodegener. 2010. PMID: 20738866 Free PMC article.
-
Pharmacological PDGFRβ inhibitors imatinib and sunitinib cause human brain pericyte death in vitro.Toxicol Appl Pharmacol. 2022 Jun 1;444:116025. doi: 10.1016/j.taap.2022.116025. Epub 2022 Apr 17. Toxicol Appl Pharmacol. 2022. PMID: 35443205
Cited by
-
The Hidden Hand in White Matter: Pericytes and the Puzzle of Demyelination.ACS Pharmacol Transl Sci. 2024 Sep 19;7(10):2912-2923. doi: 10.1021/acsptsci.4c00192. eCollection 2024 Oct 11. ACS Pharmacol Transl Sci. 2024. PMID: 39421660 Review.
-
Mural Cells: Potential Therapeutic Targets to Bridge Cardiovascular Disease and Neurodegeneration.Cells. 2021 Mar 8;10(3):593. doi: 10.3390/cells10030593. Cells. 2021. PMID: 33800271 Free PMC article. Review.
-
Transferrin receptor-targeting property of pabinafusp alfa facilitates its uptake by various types of human brain-derived cells in vitro.Front Drug Deliv. 2023 Jul 3;3:1082672. doi: 10.3389/fddev.2023.1082672. eCollection 2023. Front Drug Deliv. 2023. PMID: 40838062 Free PMC article.
-
THEM6 is a prognostic biomarker for breast cancer and is associated with immune infiltration.Sci Rep. 2023 Dec 11;13(1):21974. doi: 10.1038/s41598-023-49379-5. Sci Rep. 2023. PMID: 38081884 Free PMC article.
-
Targeting NLRP3 inflammasome for neurodegenerative disorders.Mol Psychiatry. 2023 Nov;28(11):4512-4527. doi: 10.1038/s41380-023-02239-0. Epub 2023 Sep 5. Mol Psychiatry. 2023. PMID: 37670126 Review.
References
-
- Bonacchi A., Romagnani P., Romanelli R. G., Efsen E., Annunziato F., Lasagni L., et al. . (2001). Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J. Biol. Chem. 276, 9945–9954. 10.1074/jbc.m010303200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

